Why is drug exclusivity knowledge crucial for generic drug launches?
Monitoring the drug exclusivity expiration timelines is just as important as patent expiration date when identifying opportunities and timing generic launches. Having access to detailed exclusivity data can reveal openings for generic entry even when patents are still active.
As top blockbuster drugs approach patent expiration, strategic ANDA filings are critical to secure early mover advantage. Download the Pharsight Digester report now for a deep dive into the patent strategies of the top 5 blockbuster drugs nearing expiration.
This article discusses how exclusivity of a drug impacts its generic drug launch. But before we dive into it, let’s review the various pharmaceutical exclusivities.
What are the different types of exclusivities granted and how do they impact the generic drug market?
Unlike patents which are granted and enforced by the U.S. Patent and Trademark Office, exclusivities are granted by the FDA upon approval of a drug. This provides exclusive marketing rights to the drug for a set period of time (even after expiration).
The main types of exclusivities include:
- New chemical entity (NCE) exclusivity: Granted to novel drugs and provides 5 years of market exclusivity following FDA approval. This protects drugs from generic competition for 5 years after approval even if the patent has already expired.
- Orphan drug exclusivity: Granted to drugs treating rare diseases affecting fewer than 200,000 patients in the U.S. It provides 7 years of market exclusivity following FDA approval.
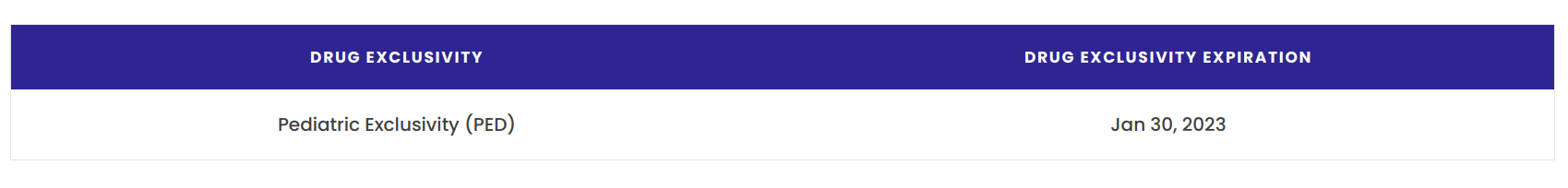
- Pediatric exclusivity: Granted if pediatric studies are conducted on a drug as requested by the FDA. It provides an additional 6 months of market exclusivity.
- GAIN exclusivity: Granted to drugs treating neglected tropical diseases. Provides an additional 5 years of market exclusivity in the U.S. following FDA approval. This is on top of any other regulatory exclusivity the drug qualifies for.
- Generics exclusivity: Also known as Abbreviated New Drug Application (ANDA) is Granted to the first generic drug applicant to challenge a brand drug’s patents and receive FDA approval. It provides 180 days of generic marketing exclusivity before other generics can enter.
But how do you keep track of which exclusivity has been approved for which drug?
Well, Pharsight, keeps track of all this and more, so you can find the perfect opportunity to launch your generic drug.
How do exclusivities delay generics?
Unlike patents which affect the ability to develop a generic, exclusivities directly prevent the FDA from approving generics for a set period of time. The NCE exclusivity in particular has the greatest impact in delaying generic entry beyond patent expiry.
For example, consider a drug with a 20-year patent set to expire on January 1, 2025. The drug received 5 years of NCE exclusivity upon approval in 2010. This means while the patent expires in 2025, the FDA cannot approve generics until 5 years later on January 1st, 2030 due to the NCE exclusivity. So the brand drug maintains exclusivity for an additional 5 years beyond the 20-year patent term.
Other exclusivities like orphan or pediatric may further extend the exclusivity period. Generics cannot even file for FDA approval until the exclusivity periods end. This creates a bottleneck where multiple generics may file on the same day the exclusivity expires.
Key differences between exclusivities and patents
While exclusivities and patents both provide exclusive commercializing and marketing rights to a company, there are some key differences:
- The U.S. Patent Office grants patents while exclusivities come from the FDA upon drug approval.
- Exclusivities apply to specific indications and conditions of use while patent protection is broader.
- Exclusivities directly prevent FDA approval while patents allow generics to file but risk infringement lawsuits.
How can generics benefit from expired exclusivity despite active patents?
The NCE-1 date is crucial for generic drug makers in the U.S. It marks the end of a five-year exclusivity period granted by the FDA for new drugs. From this date, generic manufacturers can file for approval, even if the main patent hasn’t expired. The expiration of exclusivity opens the door for these manufacturers to challenge other non-expired patents, possibly entering the market earlier than the primary patent’s expiration.
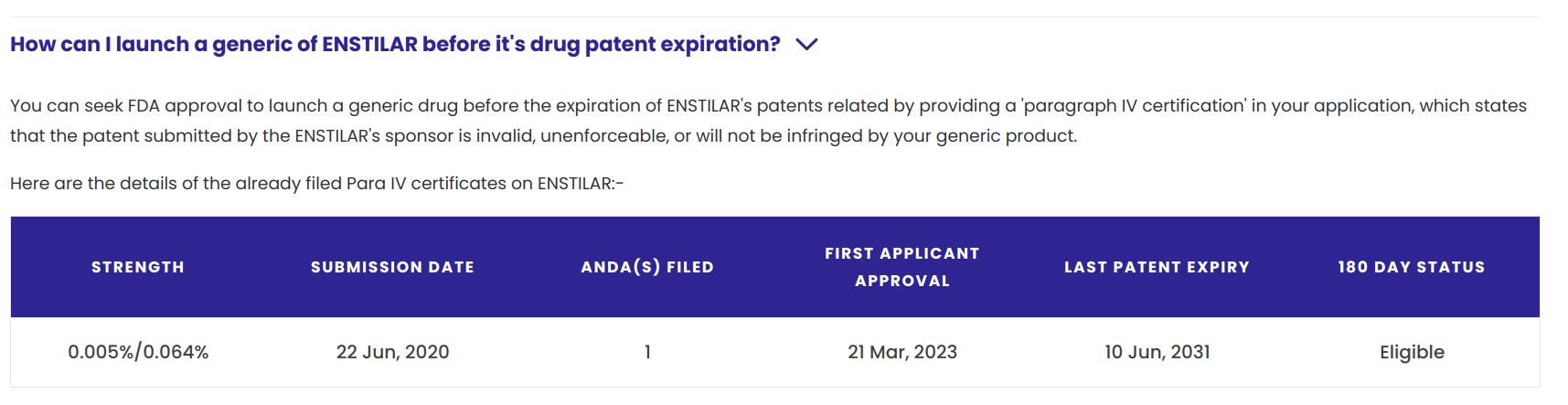
This year, Glenmark Pharmaceuticals received final approval from the US Food & Drug Administration (USFDA) for its generic version of Leo Pharma’s Enstilar, which contains the active ingredients calcipotriene and betamethasone dipropionate in a foam formulation. Glenmark stated in a press release that while the last qualifying patent for Enstilar expires in June 2031, pediatric exclusivity expired on January 30th of this year, paving the way for FDA approval of Glenmark’s generic foam.
On the lookout to launch a generic drug?
Pharsight offers detailed data on drug exclusivities and patents. Monitoring upcoming exclusivity expirations can reveal opportunities for generics even with active patents. Access a year-wise list of NCE exclusivities expiring, here.
Get the list of all the drugs with patents expiring in 2023 that are eligible for filing an ANDA, here.
Or even better, try Pharsight today!
Authored By – Nikhil Kaushal, Product Development Team




