In August 2025, the Microbiome Therapeutics Innovation Group (MTIG) petitioned the FDA to end the “IND loophole”. It’s a policy that allowed doctors to use unapproved stool-based (gut bacteria) treatments like fecal transplants under relaxed safety rules.
The industry itself asked the FDA to tighten these standards after reports of serious infections and deaths from unvetted treatments.
Now, all microbiome treatments must meet the exact, strict FDA requirements for safety, effectiveness, and manufacturing quality before reaching patients.
This decision is crucial now, as the scope of microbiome-related therapies is expanding beyond gut and digestive conditions. These therapies are now also being targeted at skin diseases, brain disorders, respiratory conditions, and metabolic disorders.
The new FDA approach will enable these diverse applications to meet rigorous safety standards, thereby building greater confidence among doctors, patients, and investors across multiple disease areas.
This regulatory clarity is also crucial for startups researching the microbiome beyond gut health. Companies with well-designed clinical trials and quality manufacturing processes will have a competitive advantage in the market.
The stricter standards also increase investor confidence by creating a more predictable path to FDA approval. This makes it easier for innovative startups to secure funding and partnerships. It signals that the microbiome treatment field is maturing from experimental medicine into a credible, mainstream therapeutic approach. The doors for innovations are opening up for startups to standardize the treatments across various non-gastrointestinal diseases.
The article covers only five startups from a list of 10 high-potential microbiome-treatment-based startups. To request the report on 10 startups, fill out the form below:
1. Parallel Health
| Founding Year | 2021 |
| Headquarter | San Francisco, CA, |
| Total Funding Amount | $2.3 Million |
| Last Funding Round | pre-seed funding |
| Website | https://www.parallelhealth.io/ |
Parallel Health is a biotech startup working on precision skin care through the microbiome. They’ve developed a patented approach that combines DNA sequencing and bacteriophage (virus) therapy to treat skin conditions.
Here’s how it works:
They take a skin sample and run it through shotgun metagenomic sequencing, which scans all the DNA present. Human DNA and irrelevant “noise” are filtered out so only microbial data remains. From this, harmful bacteria or fungi are identified—like Cutibacterium acnes (linked to acne), Staphylococcus aureus (linked to infections), or Malassezia (linked to dandruff and other issues).
Once identified, they design a customized mix of bacteriophages—viruses that specifically attack these microbes. This personalized therapy targets and destroys the problematic organisms while leaving the rest of the skin microbiome intact.
The startup created a flagship product, MD-03 Protocol, which delivers personalized skincare through telehealth. In this test, every user begins with a Skin Discovery, or a Body Blemish or Scalp Health Test for targeted areas. Parallel Health sends them test kits, including swabs, gloves, and a prepaid return envelope. After swapping, samples are sent back to the lab, where they are tested using the proprietary technology.
The customer first receives the test report and completes an onboarding call or medical consultation with a specialist. Based on the test results, customers get customized phage serums that treat conditions like acne, rosacea, eczema, and body odor.
The protocol has received positive customer feedback. One user said:
“I was hesitant to use phages, but it worked for my friend who has terrible acne. Got a test and then my first serum. Texture is nice and I like that it contains liposomal vitamin C with the phage cocktail. Whenever I feel a breakout coming on, I use it, and my spots disappear in 24 hours!! This product is amazing. I always keep it in my gym bag for after workouts, too.”
Parallel Health was selected as one of six finalists in TechCrunch Disrupt 2023‘s Startup Battlefield 200. This selection recognized their innovative potential. The company successfully raised approximately $2.3 million in pre-seed funding to fuel growth in biotech-forward skincare.
The company raised ~$2.3 M in three funding rounds and was backed by 10 investors.
2. Ibiome Biology
| Founding Year | 2021 |
| Headquarter | Hefei, Anhui, China |
| Total Funding Amount | $13.8 Million (CN ¥100 Million) |
| Last Funding Round | Seed |
| Website | m.ibiome.com.cn/en/ |
Ibiome Biology is a Chinese startup developing microbiome-based treatments using live bacteria and synbiotics (bacteria + supportive ingredients). Their work focuses on improving gut and immune health.
One of their patented solutions is a new strain of Bifidobacterium bifidum that shows strong anti-obesity and anti-diabetes effects. It helps regulate fat metabolism and insulin control, making it useful for probiotics, medical foods, or even medicines.
In early animal studies, obese and diabetic mice given this strain lost weight, had less body fat, and showed lower blood sugar and insulin levels.
The treatment seems to work by activating GPR120, a receptor that regulates glucose and energy balance, and by boosting enzymes that break down fat (ATGL and HSL).
Ibiome’s team has scientists from Yale University, Harvard Medical School, and other top international universities. The startup has published research in high-impact journals, such as Immunity. This gives Ibiome a strong academic credibility.
Its current and future pipeline includes multiple clinical-stage candidates, such as IBAI-05 for IBD and IBIO-03 for colorectal cancer. It is also researching areas such as anti-obesity and brain conditions.
The startup has raised approximately $13.8 million in early-stage venture capital financing. The two funding rounds, completed in late 2021 and late 2023, involved investors like Walden International and Hefei Venture Capital.
3. MarvelBiome, Inc
| Founding Year | 2019 |
| Headquarter | Woburn, Massachusetts, United States |
| Total Funding Amount | $3.1 Million |
| Last Funding Round | Seed |
| Website | https://www.marvelbiome.com/ |
MarvelBiome is a microbiome treatment startup that develops innovative therapies for brain, nervous system, and gut-brain axis-related conditions using bacterial strains. The startup holds multiple global patents for treatments in brain disorders, metabolic disorders, eye disorders, and other conditions using microbiomes.
MarvelBiome was founded by researchers associated with Massachusetts General Hospital and Harvard University. The founding team includes Dr. Gary Ruvkun, Dr. Rudolph Tanzi, Priti Chatter, Dr. J. Amaranath Govindan, and Dr. Elam Jayamani.
Co-founder Dr. Gary Ruvkun is a recipient of the 2024 Nobel Prize in Medicine for his role in the discovery of microRNA and its role in post-transcriptional gene regulation.
A recent patent from MarvelBiome describes the use of a specific mix of beneficial bacteria strains to treat or prevent ALS (amyotrophic lateral sclerosis). The formula combines several beneficial bacteria, including Lactobacillus plantarum and Bifidobacterium breve.
The treatment works by modulating or maintaining the body’s bacterial balance to support brain health. It can be made into capsules, liquids, injections, or gummies and delivered through methods like oral, brain injection, intravenous, or skin application.
MarvelBiome raised $3.1 million in seed funding in November 2020—investors, including DRADS Capital and C2I Accelerator, back it.
The startup claims that one of its leading drug candidates has completed the Phase 1 study, and its Phase 2 trials for brain degenerative disease are expected to begin in mid-2025. It is also conducting preclinical work in multiple disease areas.
4. Resbiotic Nutrition
| Founding Year | 2021 |
| Headquarter | Vestavia Hills, Alabama, United States |
| Total Funding Amount | $7.1M |
| Last Funding Round/Amount | Undisclosed/1.5 Million |
| Website | https://resbiotic.com/ |
Resbiotic Nutrition is a spinoff from the University of Alabama’s Microbiome Lab. The startup develops microbiome-based health supplements for respiratory and gut health using its university research.
The startup focuses on the gut-lung axis, targeting respiratory health, and extending microbiome treatments to chronic lung conditions.
One of Resbiotic’s patented innovations is on health supplement blends combining probiotic bacteria with herbal extracts, such as turmeric or other plant-derived ingredients. It balances the gut-lung connection and supports lung health with respiratory and immune benefits.
The formula delivers their three proprietary strains of Lactobacillus spp. (L. plantarum, L. acidophilus, and L. rhamnosus) to the gut lining to reduce inflammation that results from or causes lung disease.
Product Offerings
This probiotic-herb blend helps reduce inflammation in chronic breathing conditions such as Chronic Obstructive Pulmonary Disease (COPD) and asthma. It uses anti-inflammatory herbs like Vasaka, turmeric, and holy basil, with prebiotic strains to reduce neutrophilic inflammation, seasonal symptoms, and support immunity.

resG prebeet® ENERGY + Prebiotic
It’s a gut-supporting blend of beetroot and resistant potato starch combined with Vitamin B12. This combination targets gut health, energy, metabolism, and weight management. ResG prebeet® is recognized as “Best All Natural Energy and Circulatory Supplement of 2024.”

This is a novel Postbiotic with ingredients, like heat-treated Lactobacillus plantarum RSB11, Chromium, White Mulberry, Fenugreek, Vitamins D3 and B12. It’s a GLP-1 supporting supplement that helps with weight maintenance.

Resbiotic launched its Gut-X Axis supplements, like resB Lung Support and resM Postbiotic, at the U.S Walmart stores in 2025. The Previous year, it introduced the products, including ResB Lung Support Probiotic and prebeet ENERGY+ Prebiotic, to General Nutrition Centers (GNC) online and offline stores.
The startup completed a one-month trial in Ireland, testing resB Lung Support in healthy volunteers and asthma patients in 2023. The results show safe, improved lung function (FEV1%), increased Short-Chain Fatty Acid (SCFA), and better breathing quality of life (QOL) scores.
They have an ongoing 2025 trial evaluating the impact of their oral supplement on metabolic health in overweight adults.
“For patients struggling with sluggish digestion or low energy, I advise taking Resbiotic’s resG prebeet ENERGY.” –Expert evaluation by clinicians.
The resM™ GLP‑1 Postbiotic product has received many positive reviews from customers as well. To quote a few,
“My cravings are less loud now.”
“I don’t feel weighed down after meals anymore,”
The startup completed its last funding round in February 2025 with $1.5 million in investment from investors.
5. Synbiotic Health
| Founding Year | 2018 |
| Headquarter | Lincoln, Nebraska, United States |
| Total Funding Amount | $10.4 Million |
| Last Funding Round | Venture Capital Funding |
| Website | https://synbiotichealth.com/ |
Synbiotic Health is a U.S.-based microbiome ingredient startup founded by top researchers from the University of Nebraska-Lincoln. The founding members include Dr. Hutkins, Dr. Andy Benson, Dr. Tom Burkey, Dr. Jens Walters, and Tim Brummels. It works on creating combinations of probiotics and prebiotics (synergistic synbiotics) that support healthy aging and gut health. For this, Snbiotic Health uses its own discovery systems called iVS and iVE.
The startup recently filed a patent for compositions and methods to treat mental health, brain, and body disorders using specific probiotics that produce γ-aminobutyric acid or GABA. It’s a neurotransmitter or brain chemical known for its calming effects.
The innovation mentioned using special brain-friendly bacteria (psychobiotics) strains like Bifidobacterium adolescentis, Limosilactobacillus reuteri, and Lactiplantibacillus plantarum to increase GABA levels in the body. These strains can be used either alone or in combination as oral supplements.
iVS-1 (Bifidobacterium adolescentis) is the main ingredient of Synbiotic Health. The startup sells it as a strong, reliable, helpful bacteria strain designed for gut barrier and health benefits. It also published research showing that iVS-1 produces high levels of GABA and folate to improve mood, sleep, and B-vitamin absorption. This strain offers dual benefits for brain and gut health.
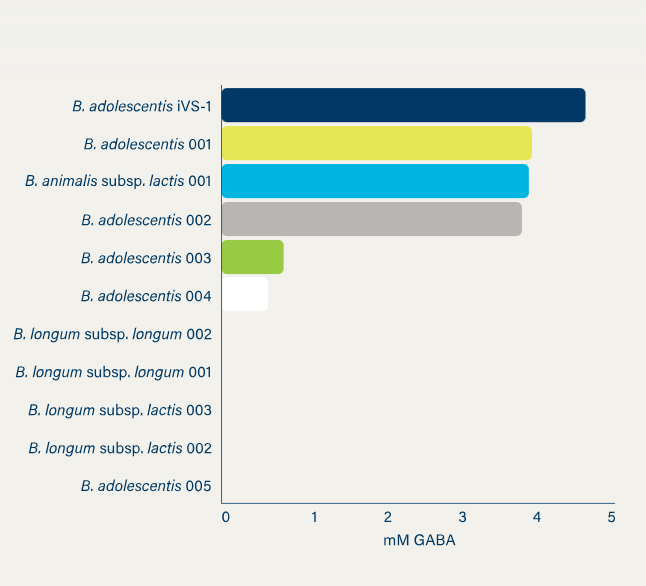
Synbiotic Health also offers the IVE Platform™. It is a high-speed testing system that helps researchers test and select many different nutrients and bacterial strains simultaneously in the lab (in vivo). It is used to build a large collection of human and pet strains with various health benefits.
IVS is another platform offered by the startup that identifies the most competitive, gut-surviving strains by testing them in real body conditions (in vitro) and selecting those that perform best.
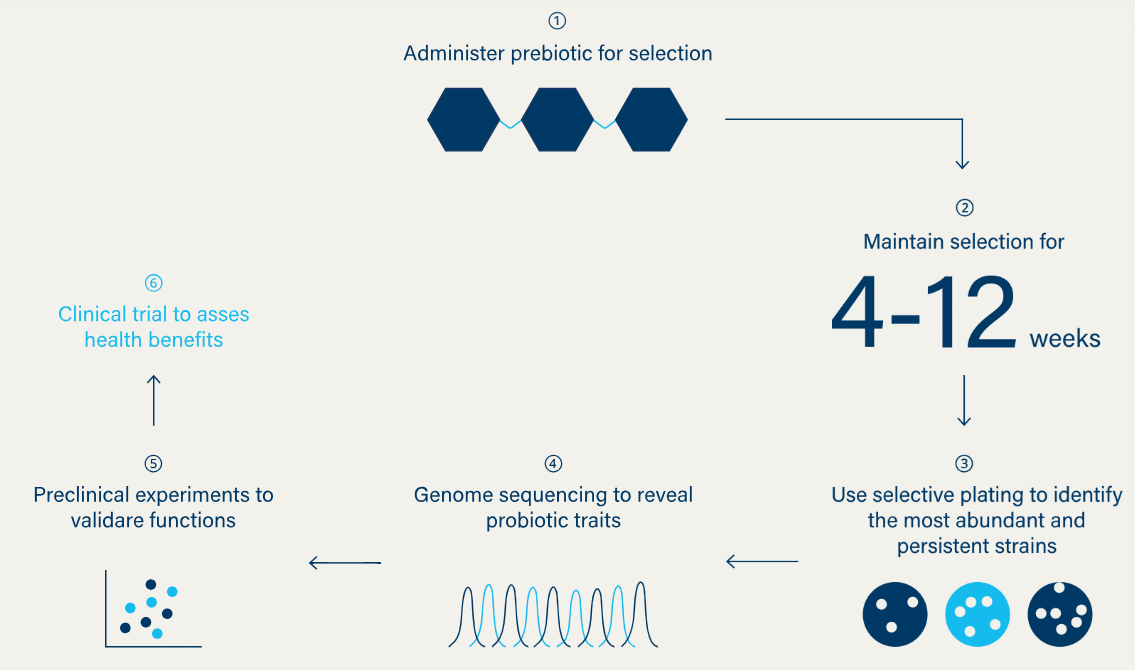
For its future pipeline, the startup claims to have over 20 strains in early trials and 8 new strains undergoing pre-FDA safety and stability testing. It also has two completed clinical trials and two studies currently being designed.
The startup announced the opening of a 43,000 sq ft R&D and production facility in late 2023. Later, it announced a spin-off, NoviaHealth LLC, which will start operating from this facility in the second quarter of 2024. This company will focus on commercializing probiotic ingredients. Its offerings include probiotic concentrates and custom blends, as well as contract-based development and manufacturing services for dietary supplements.
Synbiotic Health has also secured $4 million in 2024 in a later-stage VC deal to support R&D and commercialization.
What’s Next
The global human microbiome market is expected to grow from $1.6 billion in 2025 to $8.9 billion by 2034. This growth comes from large, targeted funding rounds for companies with precision medicine platforms.
For example, SNIPR Biome, a company creating CRISPR-based microbial gene therapy treatments, successfully raised €35 million in Series B financing on August 7, 2025. Similarly, MRM Health, which focuses on treatments for inflammatory diseases and immune-oncology, raised €55 million in Series B funding to advance its programs.
Market growth is not limited to high-value treatments alone.
A significant business deal from this period shows the dual nature of the market’s expansion. On March 21, 2025, PepsiCo announced a definitive agreement to acquire Poppi, a fast-growing prebiotic soda brand, for $1.95 billion.
A health-focused biotech startup and a mainstream consumer packaged goods giant are both making significant financial moves in the same space. It proves that the microbiome is no longer just a niche scientific topic.
The landscape suggests that companies focusing on Microbiome-based non-gut disease treatments have generated increased investor interest. These players are widening the microbiome-based therapies beyond gut health with investor support and clearer regulations.
However, this expansion comes with a challenge for R&D teams to keep up with shifting regulations and innovations in the Microbiome therapies landscape.
That’s where GreyB can help you.
Our experts can help you keep an eye on the latest startup activities, competitor moves, innovations, patent filings, and regulatory changes. Find the answers to questions and challenges that R&D teams are not even aware of yet:
How Can We Help You?
We support industry-leading R&D and Innovation professionals through complex problems. Describe your challenge, and let us bring clarity and expertise.