US9320716– An apparent sword in the arsenal of Cosmo Technologies -a subsidiary of COSMO Pharmaceuticals- has been on the battlefield a lot, lately.
This weapon of choice, both individually and in combination with others, has been used to rage lawsuits against big pharma companies including the likes of – Sun Pharmaceutical, Mylan Pharmaceutical, Lupin Limited, Alvogen group, Allergan Plc for reason cited as an infringement.
With IPR Reviews (Read Invalidating the patent) being the latest tactic in the book to minimize the chances of royalty payments, Mylan Pharmaceutical filed a petition with PTAB in March 2017 challenging the patentability of ‘716 patent.
Now, though the petitioner provided a handful of references challenging the validity of US’716, we conducted a prelim analysis on our side, and believe the petitioner could cite more references to killing this patent than what they have cited now.
First, let’s have a look at the patent.
What is US ‘716 all about?
The ‘716 patent generally claims the composition of a well-known drug – budesonide (BTW, If you think it’s new, this paper published in 1994 would burst the bubble) – for the treatment of inflammatory bowel disease like Crohn, a disease that budesonide was known to treat from a long time.
So what is new in this patent –
It claims a controlled release oral pharmaceutical composition comprising:
– budesonide in an amount effective to treat intestinal inflammatory disease;
– a macroscopically homogeneous structure in a tablet form that controls the release of budesonide comprising:
- at least one lipophilic compound e. attraction for lipids and
- at least one hydrophilic compound e. attraction for water
– a gastro-resistant coating on the macroscopically homogeneous structure that prevents the release of budesonide in the stomach.
In all, ‘716 patent provides a composition that allows the controlled release of budesonide drug on the affected parts of the body. It has three independent claims – 01, 12, and Claim 21. With Claim 1 reciting all the features mentioned above, Claim 12 requires “at least one amphiphilic compound” i.e. having attraction for both water and lipids rather than “at least lipophilic compound” and Claim 21 requires “at least one amphiphilic compound” and “at least lipophilic compound”.
The ‘716 patent is claiming priority from two Italian applications MI2000A000422 and MI99A001317, filed on March 3, 2000, and June 14, 1999, respectively.
Why do we feel the claims can be challenged?
If we look at the patent, it seems to be the combination of two essential features- the composition of lipophilic and hydrophilic compound to control the release of the budesonide and a gastro-resistant coating to prevent the release of budesonide in the stomach.
Based on our preliminary analysis, we found a paper “Budesonide for inflammatory bowel disease” by David B. Sachar, published in 1994 which mentions the delay-release coating on the budesonide that aid in the delivery of the drug to inflamed regions of the bowel. Below we have highlighted a relevant portion of this paper-
We found another paper, Meta-analysis vs Mega-analysis by Lorinda Simms published in 1998, citing the above paper that consolidated our belief that the coating is done to deliver the drug to the inflamed portion directly. Here is the relevant excerpt from this paper –
“The ideal corticosteroid for the treatment of Crohn’s disease should possess high anti-inflammatory activity in the area of the bowel which is inflamed but not cause systemic steroid side effects (Sachar. 1994: Spencer 8 McTavish. 1995). Oral budesonide (Entocort CIRB. Astra Draco AB. Lund. Sweden) has been designed to deliver the drug to the terminal ileum (small intestine) and proximal colon (large intestine). The oral budesonide tablet has two delayed-release coatings, ethylcellulose and an acrylic-based resin (Eudragrit LI 00-55Q) which aid in the delivery of the drug to inflamed regions of the bowel (Sachar, 1994). Following absorption, budesonide undergoes rapid metabolisrn in the liver to inactive rnetabolites. The rnetabolites of budesonide have little or no corticosteroid effects (Spencer & McTavis h. 1 995)”
There are more such papers published before 1999 that disclose such coatings to directly deliver the drug to the inflamed portion of the bowel, another such paper being A cortisol suppression dose-response comparison of budesonide
On dwelling deeper into the analysis, we found a patent US5858998A filed in Sep 1997 by German company Dr. Falk Pharma that talks about the homogeneous composition of the tablet, including lipophilic and hydrophilic compounds.
 Here is the relevant text from this reference –
Here is the relevant text from this reference –
“Oral preparations of the pharmaceutical compositions of the invention such as by tablets, capsules, powders, liquids such as suspensions, solutions or emulsions or as a syrup are preferred. When budesonide is formulated as a tablet, usual carriers and excipients such as lactose, microcrystalline cellulose, starch and anhydrous silica, lubricants such as hydrated castor oil, magnesium stearate, sodium lauryl sulfate and talc as well as binders such as starch, glucose, gum arabicum and mannitol are used”
On further exploring the patents from this German company, we found quite a close patent US5932249A which seems to describe budesonide tablets with a controlled release profile. These tablets include the composition of microcrystalline cellulose (macroscopically homogeneous structure) which include cellulose compound or methacrylic acid (hydrophilic compounds) and magnesium stearate (lipophilic compound). Further, this composition is layered with compound poly(methacrylic acid, methyl methacrylate) which are insoluble in gastric fluid i.e. gastro-resistant.
Below is the excerpt of the relevant section from this reference, particularly example 7 –
“[Claim 01]
Budesonide pellets with a controlled release profile, where the release of active substance is 0% after 15 minutes and at least 90% after 120 minutes, which pellets comprise, from the inside to the outside: a) sugar spheres; b) an active substance layer comprising micronized budesonide and one or more water-soluble auxiliaries; c) a first lacquer layercomprising 80 to 97% of at least one lacquer which is insoluble in gastric fluid and soluble in intestinal fluid and 3 to 20% of at least one lacquer which is insoluble in gastric and intestinal fluids; and, d) a second lacquer consisting of 100% of at least one lacquer which is insoluble in gastric and intestinal fluids.
[Claim 06]
The pellets of claim 1 in the form of capsules, tablets or granules.
[Claim 13]
The pellets of claim 1, wherein the first lacquer layer comprises (i) a poly(methacrylic acid, methyl methacrylate) with a molar ratio of 1:1 and a molecular weight of 135,000 or a poly(ethyl acrylate, methacrylic acid) with a molar ratio of monomeric units of 1:1 and a molecular weight of 250,000;
The invention is explained in detail by means of the following examples.
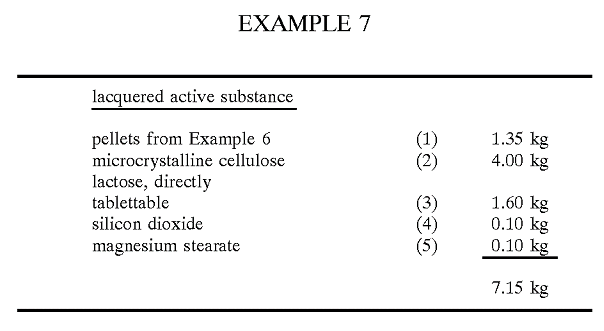 Components (2) to (4) are passed through a screen With a mesh Width of 1.0 mm, and then component (1) is added and mixing is carried out for 10 minutes. Subsequently component (5) is added, and mixing is again carried out for 5 minutes. The mixture is compressed to tablets With a diameter of 13 mm. The Weight of the tablet is 715 mg, and the budesonide content is 1 mg. The tablet disintegrates after 2 minutes, releasing the lacquered budesonide active substance pellets”
Components (2) to (4) are passed through a screen With a mesh Width of 1.0 mm, and then component (1) is added and mixing is carried out for 10 minutes. Subsequently component (5) is added, and mixing is again carried out for 5 minutes. The mixture is compressed to tablets With a diameter of 13 mm. The Weight of the tablet is 715 mg, and the budesonide content is 1 mg. The tablet disintegrates after 2 minutes, releasing the lacquered budesonide active substance pellets”
Similarly, we found another reference WO1997044021A1 published in 1997 that describes the anesthetic oral drug having the composition of hydrophilic and lipophilic compounds and it is enteric coated which remains intact in the stomach for treating Inflammatory bowel disease. This provides another motivation for any drug to be delivered in a controlled manner to the inflammatory bowel as discussed in US’716.
All these pieces of evidence are just a tip of the iceberg, we feel more strong prior-art can be identified by spending more time on the analysis. Besides, we feel that there are other pretty potential areas that can be searched and were overlooked by the examiner during the prosecution.
Some of these areas include searching in the Journals and clinical trials like the New England Journal of Medicine, Journal of Clinical Epidemiology, British Medical Journal Clinical Research Edition, PubMed, et cetera. These journals from the 90s regularly publish the reports of the clinical trials and new methods discovered for delivering drugs. Some of the initial pieces of evidence that we uncovered are from these Journals and more could be found if some more time is spent on the search.
Dig Deep is The Mantra to Invalidate this Patent.
Authored by: Nikhil Gupta, Manager, Search Team, and Srijeet Majumdar, Research Analyst.











