With companies filing for ANDAs, the global prescribed generics sales are expected to reach nearly 100 billion U.S. dollars by 2026.
One example of this is Rexulti – a prescription medicine used along with antidepressant medicines to treat major depressive disorder (MDD) in adults.
Zydus Pharmaceuticals stood out by being among the first applicants to submit a comprehensive Abbreviated New Drug Application (ANDA) with a “paragraph IV certification” for these tablets. This granted them a 180 day of shared generic drug exclusivity.
Thus, giving Zydus an edge over competitors like Lupin, Amneal, and Ajanta Pharma, who were a little behind in filing for ANDA approval.
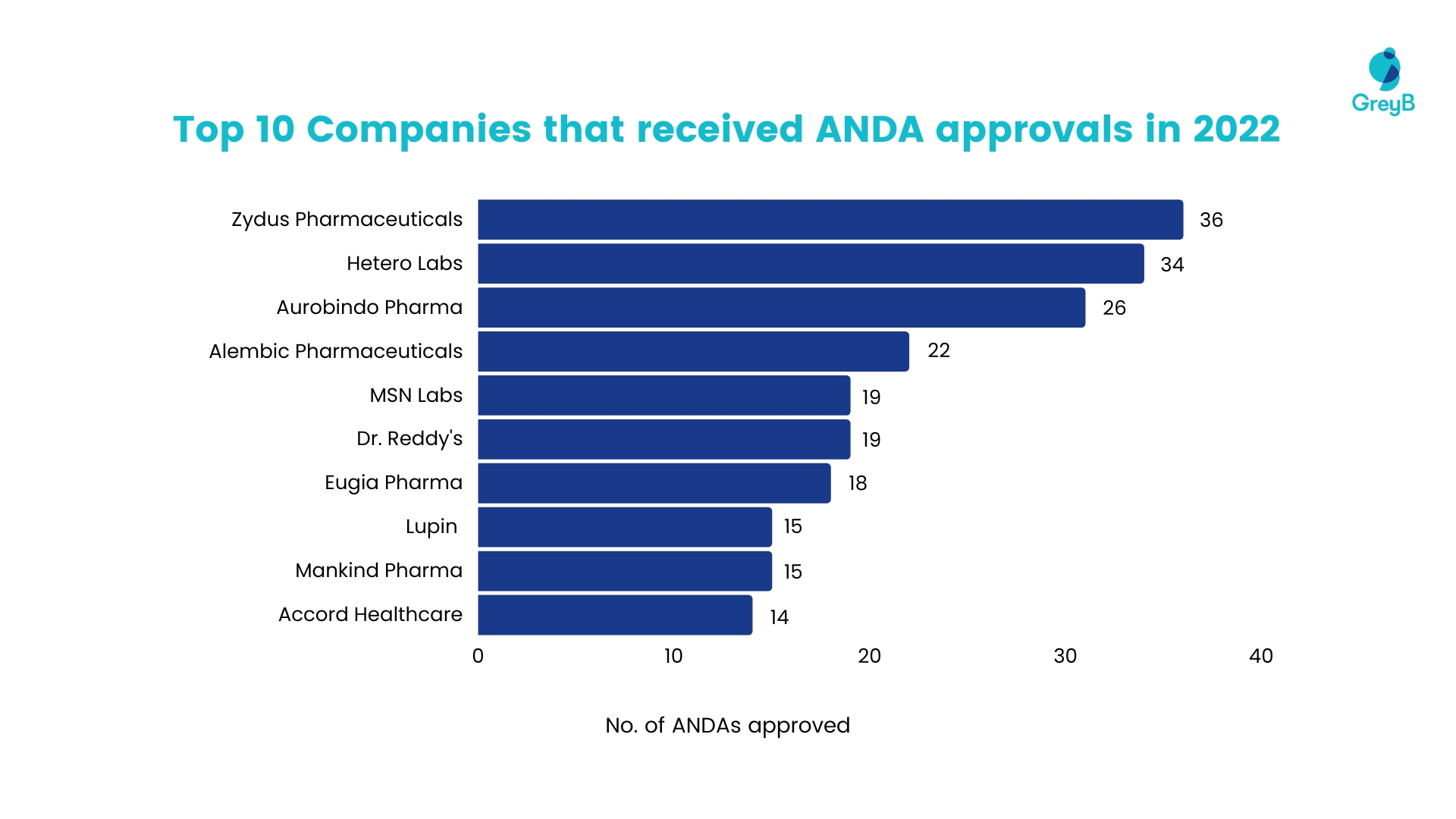
In 2022 alone, the U.S. Food and Drug Administration (FDA) either approved or tentatively approved 914 Abbreviated New Drug Applications (ANDAs), including 106 brand-new generics.
As top blockbuster drugs approach patent expiration, strategic ANDA filings are critical to secure early mover advantage. Download the Pharsight Digester report now for a deep dive into the patent strategies of the top 5 blockbuster drugs nearing expiration.
Want to file an ANDA before the launch of your next generic in the coming years? If yes, then keep reading.
Steps to follow to launch the generic
1. Identify the right opportunity
To secure the initial generic ANDA approval, you must identify a drug in high demand that will soon lose its patent protection and exclusivity. Tracking these expiring patents isn’t just a matter of staying informed, it’s a strategic move to maintain your competitive edge.
Failing to monitor and act upon the nearing expiration of patents could result in your competitors seizing the opportunity and gaining a significant advantage. Imagine the potential loss of market share and revenue if another company gets ahead of you in bringing a generic version of a sought-after medication.
Stay ahead of the curve and maintain your position as a leader in the market by strategically identifying and pursuing drugs with expiring patents.
How?
Well, to get you started, here is a list of patents expiring in 2024.
Want to plan ahead in the future? How about getting a list of patents expiring as late as 2032?
Well, on Pharsight you can not only have the complete drug patent expiration list but also set up notifications for everytime a drug patent expires!
2. Conduct a feasibility study
Upon identifying a potential opportunity, the next step involves conducting a feasibility study to determine its viability. This includes analyzing the following factors-
Market Potential: Evaluate the demand for the generic drug within the market and gauge the level of competition you’ll face while launching the drug. Understanding the market’s size and growth prospects is crucial in assessing the potential success of the generic drug in terms of revenue and sales.
Manufacturing Costs: Scrutinize the estimated expenses associated with manufacturing the generic drug. Consider factors such as raw materials, production procedures, packaging, and quality control measures. Lower manufacturing costs can facilitate competitive pricing and improved profit margins.
Patent Expiration Dates: Identify the expiration dates of patents associated with the branded drug. The optimal timing for filing an Abbreviated New Drug Application (ANDA) is after the patent has expired, allowing generic manufacturers to enter the market legally.
Previous Litigations: Investigate past patent litigations or legal disputes linked to the reference drug. A comprehensive understanding of previous legal conflicts aids in evaluating potential risks and obstacles that might emerge during the development and marketing of the generic drug.
Regulatory Requirements: Familiarize yourself with the regulatory requirements established by the FDA for ANDA approval. Adhering to these regulations is essential for obtaining regulatory approval for the generic drug. Gain a clear understanding of the essential documentation, data, and testing requirements necessary for submission.
By thoughtfully addressing these factors in a feasibility study, one can make informed decisions and enhance the chances of success for the generic drug.
3. File an abbreviated new drug application (ANDA)
To be the first one to submit a generic drug, you have to file a shorter application called an Abbreviated New Drug Application (ANDA) to the FDA. To get the FDAs approval, your ANDA should prove that your generic drug is just as safe, does the work, and is of the same quality as the original brand drug. You’ll also have to inform them how you make the drug and show that you can produce it at scale.
4. Get your application reviewed by the FDA
Once you have submitted your ANDA, the FDA will review it to ensure that your generic drug meets all the regulatory requirements. This includes evaluating the drug’s safety, efficacy, and quality and reviewing your manufacturing process.
5. Launch your generic drug
If the FDA approves your application, you can launch your generic drug. This can be a highly-profitable opportunity, as patients and healthcare providers always seek cheaper alternatives to brand-name drugs.
Therefore, if you want 180 days of exclusive access before your competitor enters the market, identifying the right opportunity is the most crucial step. Timely alerts of an expiring branded drug can lead you to become the first ANDA filers.
Concluding Notes
Monitoring large amounts of soon-to-expire patents can be a massive task that if done manually, might increase the chances of human error. This might lead to you losing not one but many golden opportunities in the generic drug market.
Well, that’s where our tool, Elixir, can help!
Why Choose Elixir?
Elixir is a powerful platform that lets you stay up-to-date on the latest drug patents and regulatory activity from USPTO, EPO, and Orange Book. With a user-friendly interface and powerful monitoring tools, Elixir makes it easy to stay informed and make data-driven decisions for your business or research.
This tool provides you with a platform where you and your team can brainstorm and work in collaboration without any interference.
It can help you conduct a feasibility study by analyzing the patent expiration dates and notify you about the patents soon reaching expiration, so you can strategize filing ANDA accordingly.
How? Well, just import the list of patents, and you are good to go!
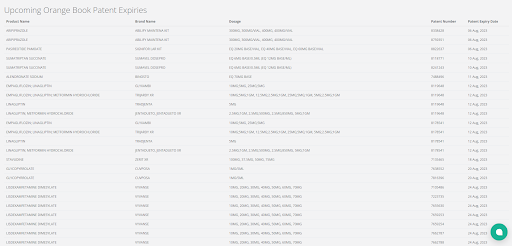
With Elixir, you’ll get frequent updates and a monthly summary of the happenings related to the patents on the list.
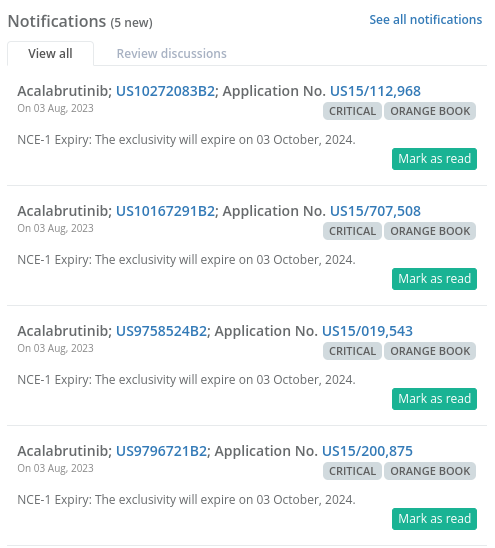
Additionally, you can evaluate a drug’s value by keeping an eye on the New Chemical Entity (NCE) exclusivity dates on Elixir. Watching these dates can help you spot valuable medicines, allowing you to make well-informed choices.
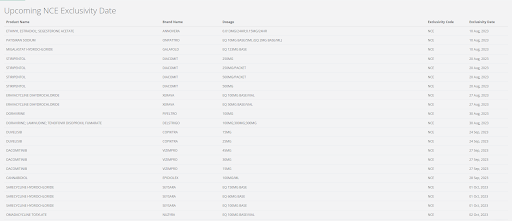
The screenshot below lists the drugs with upcoming NCE Exclusivity dates.
Elixir further helps you keep a timepiece on –
- File History
- PTAB trials
- Orange book Updates,
- SPC Updates
- Third-Party Opposition filed etc., and helps in identifying market entry opportunities for making informed management decisions
- Tracking Divisional and Continuations
- Tracking US reissued patents
- Monitoring 21 countries, including the US, EP, Russian Federation, Singapore, Japan, Philippines and many more.
Elixir will give you a head start in the market and increase your chances of being the first to submit an application and get the ANDA approval.
Want to stay ahead of the game? Don’t wait! Try Elixir today.
Authored by – Smiksha Sood, Product Development Team
Edited by – Ridhima Mahajan, Marketing
Also Read – Lead the generic drug market with this one effective strategy