The recent FDA controversy around Novo Nordisk’s Ozempic has brought many eyes to the drug.
For the uninitiated, Ozempic (semaglutide) is an anti-obesity medication for long-term weight management. However, an FDA-approved label was recently updated to warn of potential serious intestinal side effects like obstruction or blockage.
In this study, we see how this FDA update will change the course for Novo Nordisk and Ozempic.
Ozempic’s Background
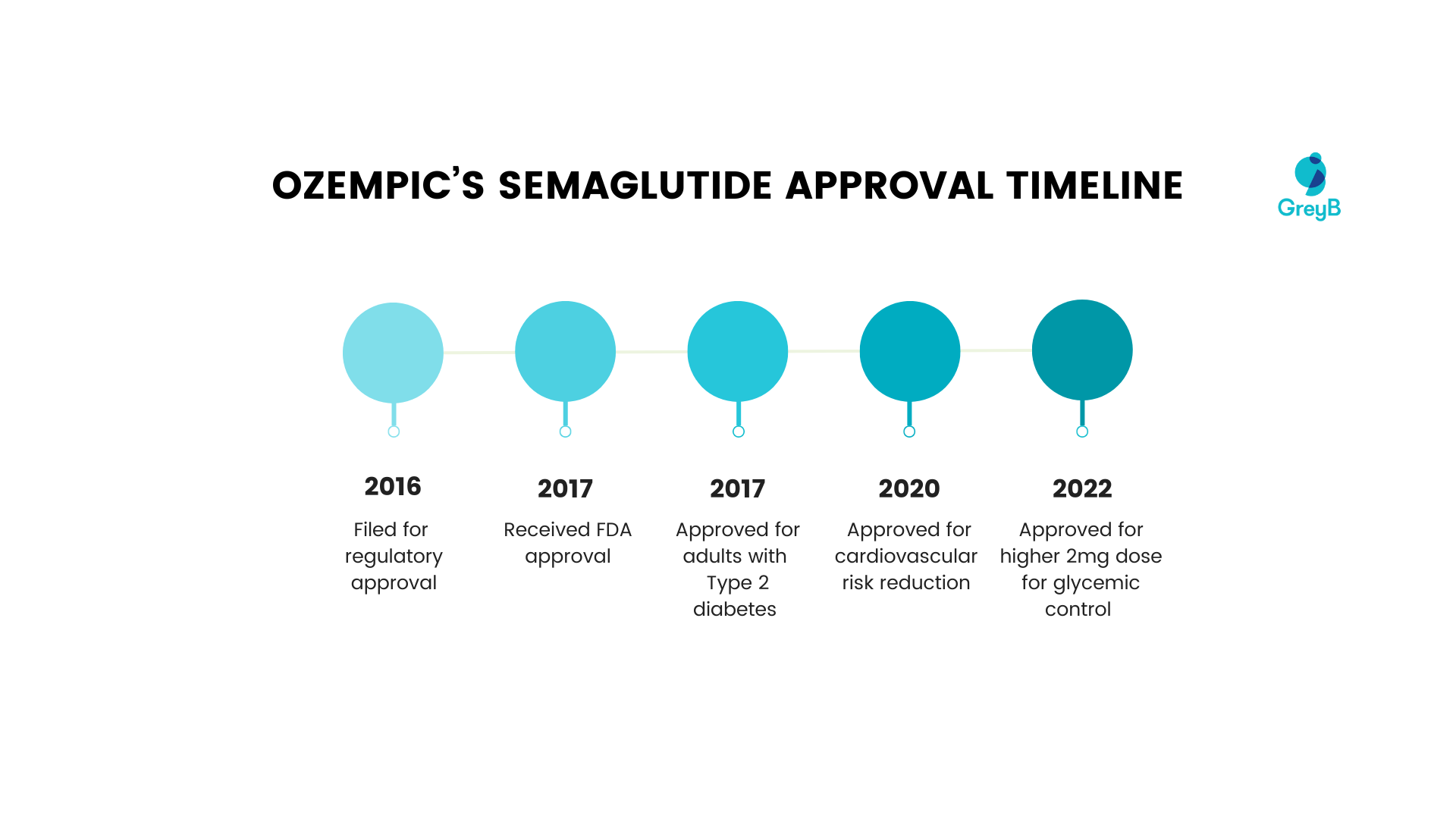
Ozempic’s development timeline
Here’s a timeline of Ozempic’s approval for different diseases by the US FDA.
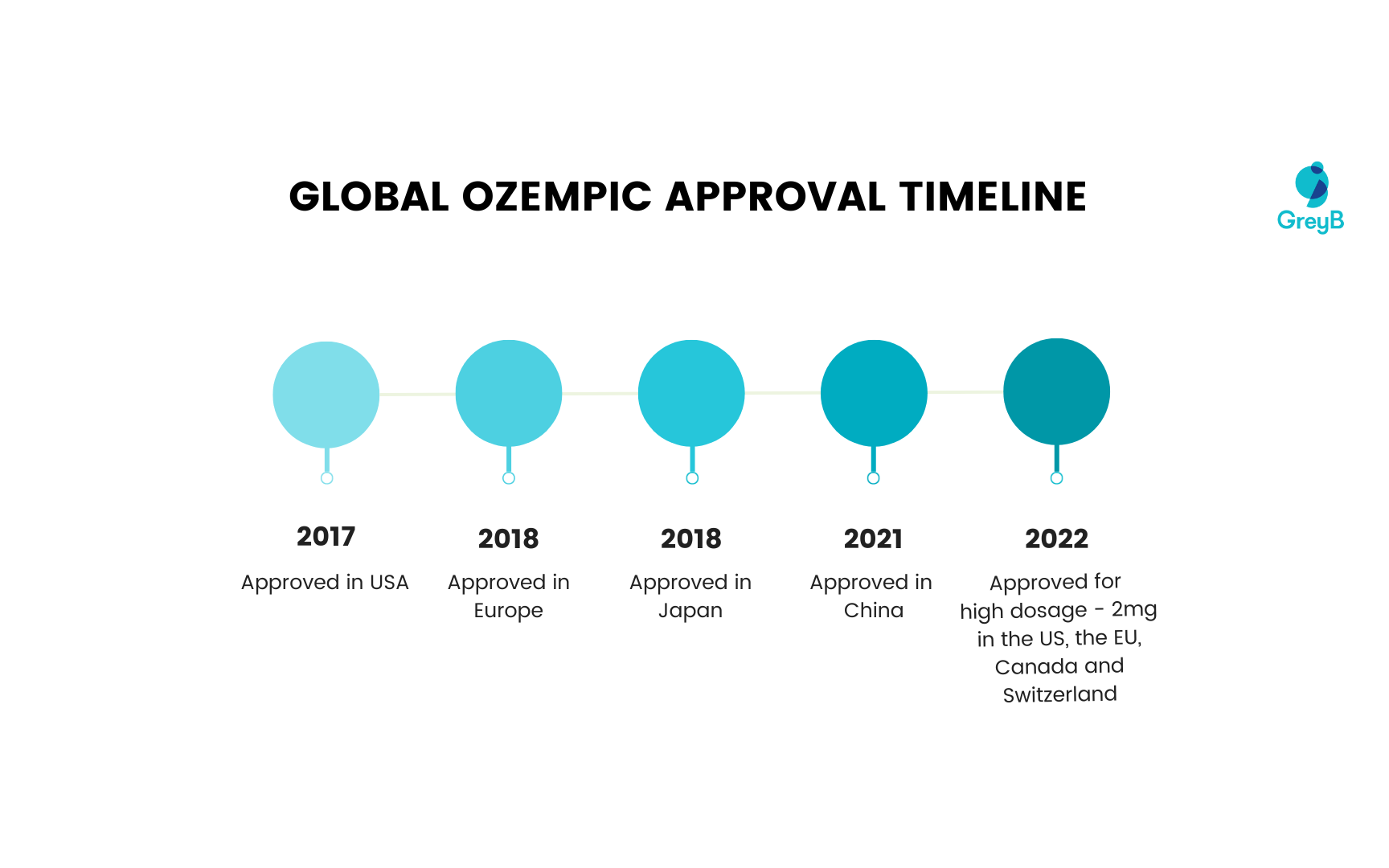
Ozempic’s global approval timeline
With its approval in 2017, Ozempic was sold globally across countries like the US, Japan, Canada, Switzerland, Germany, Netherlands, Sweden, UK, Australia and France.
Here’s a timeline of Ozempic’s approval in different countries.
Why is Ozempic Important to Novo Nordisk?
Ozempic holds great importance for Novo Nordisk due to its remarkable growth prospects. The leading drug is anticipated to achieve a 23% sales increase, reaching $12.5 billion in 2023.
This not only consolidates Ozempic’s position as the dominant market leader but also signifies projected sales that are 54% higher than those of its closest competitor, Trulicity (dulaglutide), by Eli Lilly, which expects sales of $8 billion. (Source)
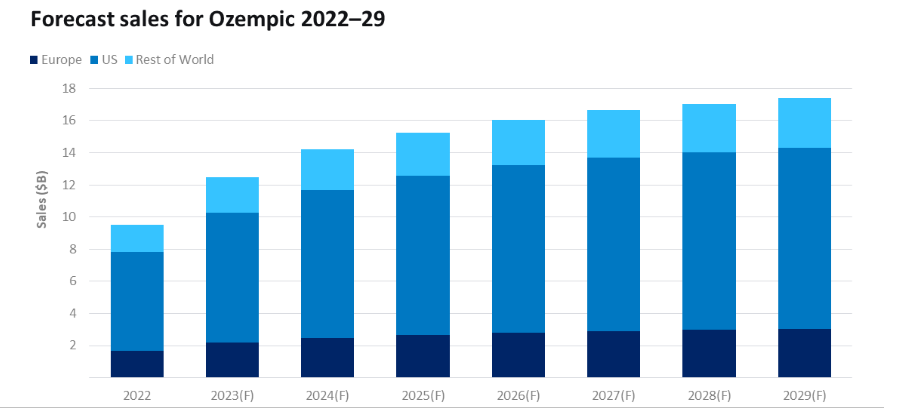
This substantial sales growth of Ozempic reaffirms its ongoing leadership in the type 2 diabetes market.
To protect its position and investment, Novo-Nordisk has strategically filed multiple patents around the drug, forming a patent thicket.
Ozempic Patent Thicket
| Indications | Obesity | Non-alcoholic steatohepatitis (NASH) | Diabetes | Alzheimer’s disease | Cardiovascular Events |
| Composition of Matter | US8129343B2 Expires December 05, 2031 | ||||
| Indication/ Method of Treatment | US20200237876A1 US20200197491A1 Under Application stage | US20210330748A1 US20220047678A1 Under Application stage | US20180085435A1 US20200164042A1 Under Application stage | US20220280612A1 Under Application stage | US20190134162A1 Under Application stage |
| Formulation | US20210252111A1, US20180169190A1, RU2803237C2, US20210236601A1, WO2015162195A1, WO2023012263A1, US20230165939A1 Under Application stage | ||||
| Manufacturing | US20220088500A1, US20220370362A1, RU2021128717A, BR112021016694A2, US20150150811A1, WO2021105393A1, US20220409701A1 Earliest Expiry: June 19, 2033 | ||||
| Other (Antibody structure, etc.) | US20200308274A1, US20160136246A1, US20140004198A1, JP07095027B2 Earliest Expiry: March 31, 2040 | ||||
As top blockbuster drugs approach patent expiration, strategic ANDA filings are critical to secure early mover advantage. Download the Pharsight Digester report now for a deep dive into the patent strategies of the top 5 blockbuster drugs nearing expiration.
With a multitude of patents in various application stages, it’s clear that Ozempic is still in its initial phase, and there’s much more to anticipate in the near future.
Given that Ozempic’s composition of matter patent is set to expire in 2031, there’s a significant time window before the generics can enter the market. However, monitoring all relevant activities is essential for Generic drug companies to ensure a smooth market entry and avoid legal challenges.
That’s where Elixir steps in.
Elixir is an all-in-one solution to stay updated on breakthrough drugs, including their application status, ongoing legal matters, and more. It keeps you informed about any developments concerning the drug patents on your watchlist.
GreyB’s Analysis
Due to recent patent disputes, the absence of Ozempic alternatives is a key concern. The court denied an extension for the semaglutide patent on May 9, 2023, and the U.S. Patent and Trademark Office’s patent trial and appeal board (PTAB) is reviewing Novo Nordisk’s patent following a challenge by Mylan Pharmaceuticals.
Further, we believe that the scarcity of Ozempic in the market may open an opportunity for the generic manufacturers to apply for an Abbreviated New Drug Application (ANDA), potentially increasing access to more affordable alternatives as the generic drug industry grows.
Though the recent FDA warning doesn’t necessarily signify the end of Ozempic’s presence in the market, however, staying vigilant and monitoring activities related to the patent is essential for strategic decision-making.
Elixir gets you a head start in the market. Thus boosting your chances of being the first to apply and gain ANDA approval, securing market exclusivity before other generic drug manufacturers.
Try Elixir today!
Authored By – Unnati Agarwal, Life Sciences
Edited By – Ridhima Mahajan, Marketing