If drug discovery is your bread and butter, you might already know that drug development often takes a decade of research and billions in investment before the drug reaches the market. In other words, the drug development process is complicated, difficult, and a rather costly business. But all hope’s not lost yet.
To detangle the process of drug discovery. Artificial Intelligence and machine learning have come as a ray of hope for the pharmaceutical industry. AI solutions allow researchers to quickly design novel drugs that display the desired properties. Many companies are working on designing novel drug molecules using these advanced technologies.
Talking about novel drugs, every year, patents on these novel drugs expire. As monitoring these drug patents is important to many pharma companies, we listed over 200 drug patents that are expiring between 2025 and 2030.
Fill out the form below to get these detailed insights delivered to your inbox:

Who are these companies and how are they using the power of AI and ML?
To find out, we dived into this technology and checked the companies providing unique solutions. We found some startups utilizing innovative ways and filing patents to solve the problems faced during the drug-discovery process. Many companies are collaborating with and acquiring different start-ups to become future leaders in this domain. In this article, we will be focusing on the start-ups that are leading and shaping this industry by employing the power of AI to discover the cure for serious human diseases.
How did we shortlist these start-ups?
To check different players from this domain, we first prepared targeted search queries which we executed on patent databases. From there, we observed that a few companies have patents on drug discovery processes using advanced technologies like AI, ML, etc. In the next step, we checked these companies’ other market activities such as-
- Collaboration/partnership for developing new solutions or licensing their technologies to other future leaders from this domain
- Funding amount received from Government, other companies, etc.
- After checking all the patent and market data, we narrowed the list to the following 10 start-ups. These startups are not just offering unique solutions to the market but are actively filing patents for their innovations.
Here are the Top 10 AI Drug Discovery Startups:
1. Exscientia
Founded in 2012, Exscientia developed an AI-powered design platform known as Centaur Chemist, which not only identifies potential new drug targets but also builds those drugs and sends them to clinical trials.
With around 14 patents filed, they’ve got a considerable IP portfolio. 7 out of these 14 patents are related to the AI domain itself. One of its recent patents – US20200013486A1 talks about an in-silico system that helps solve the real problems of the industry, which is lead optimization and identification of drug effectiveness. Now, if I take the first step in the drug discovery process- to select a chemical moiety and optimize its effectiveness, performing this task manually can be a tedious and lengthy process. But this patent proves an innovative way of lead molecule optimization via –
- True lead optimization, by assessing large numbers of hypothetical molecules and converging on a more limited number of potentially active molecules, thereby reducing the number of compounds that must be synthesized and tested during a project.
- Prediction of drug candidates having desired biological properties and activities.
It simplifies the de novo drug design process, and the costs of lead optimization are reduced, thereby having a direct impact on drug discovery.
The company has recently announced its first AI-designed molecule for treating cancer using the body’s own immune system, co-developed with Evotec. It will be entering human clinical trials soon. This happened following its 2020 announcement of bringing an AI-designed molecule for treating obsessive-compulsive disorder (OCD) to a phase 1 clinical trial in partnership with Japanese collaborator Sumitomo Dainippon Pharma.
Explore more in drug discovery
Acquisition/Mergers and Collaborations
Exscientia has acquired Allcyte, an Austrian company developing an AI platform to predict how well cancer treatments will work in individual patients. Allcyte’s technology relies on deep learning to analyze the effects of various drugs on live samples of an individual patient’s tissue, rather than on artificial or animal models. By using allcyte’s platforms, Exscientia will be able to take a precision medicine approach to design drug molecules, ensuring that they’re even more effective at targeting tumor tissues than those designed with Exscientia’s technology alone.
Recently, the company also entered into a joint venture with GT Apeiron Therapeutics (Apeiron), a Shanghai-based company, to provide its AI-driven drug identification and design capabilities to accelerate the discovery of a therapeutic drug.
EQRx and Exscientia also teamed up on discovery-through commercialization to bring cheaper medicines to patients faster. As part of the deal, Exscientia will pick up discovery duties while EQRx will handle development and commercialization.
Where is the startup receiving its funding from?
In March 2021, Bill Gates-backed Oxford biotech raised $100M in funding to discover new drugs using AI (source).
In April 2021, Softbank led a $525m round in British drug developer Exscientia (source).
2. Standigm
South Korean start-up, Standigm, founded in 2015, discovers drugs using AI, saving time and cost when compared to traditional methods. Their AI platform, Standigm BEST, intends to explore latent chemical space to generate novel compounds with desired properties. Other than chemical data, they also analyze biomedical literature to speed up de novo drug design.
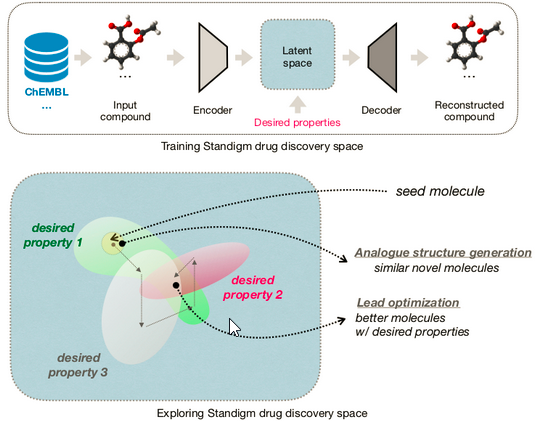
Once candidates are identified, Standigm Insights provides biological interpretations to discover pathways, and therapeutic patterns and prioritize potential targets. The startup’s solutions eliminate uncertainty in the drug discovery process to save time and costs during development.
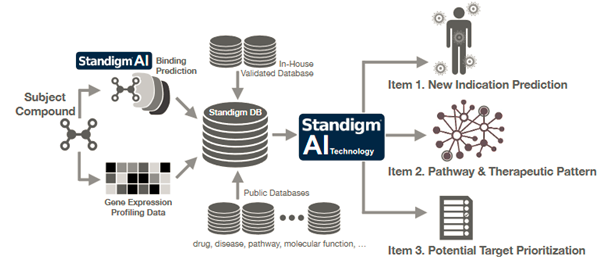
In a nutshell, Standigm has achieved the automation of the whole drug discovery process based on their AI platforms, including Standigm ASK™ for target discovery, Standigm BEST™ for lead design, and Standigm Insight™ for drug repurposing. To date, Standigm has run 22 in-house or collaborative drug pipelines using workflow AI technology.
The startup has filed a total of 10 patents. Given below are some of the interesting patents related to their innovative technology-
- KR2018022537A – This patent is mainly focusing on a method that utilizes machine learning in predicting the effects of the drug combination. This method uses a computer algorithm to collect various data given below. All this collected data enables the prediction of the therapeutic efficacy of a new combination drug when synergic effects are expected according to the drug combinations. This method helps in finding out which targets or proteins are affected by which drugs. And how they interact if used in combination for the treatment purpose.
- cell-related data
- drug-related data and,
- drug/cell correlation-related data
- KR2020145835A – This patent talks about a thioridazine composition used to treat or prevent metabolic liver disease, steatosis, inflammation, reduces fibrosis, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, etc. Further, the thioridazine molecule is selected through a screening method using artificial intelligence (AI) deep learning technology after confirming its therapeutic efficacy.
- WO2021080295A1 – This patent describes a method and apparatus for generating an effective compound structure by using the learning data of existing biologically active structures. The method identifies a candidate group of effective compounds through artificial intelligence based on existing compound data. Using this data, the AI system can derive a new type of compound having properties related to the target effective molecule and can simulate it. In simple words, this AI-based molecular structure design method is automatically designing the molecular structure of a new drug candidate by selectively modifying only a part of a given material structure by using the existing compound data.
Where is the startup receiving its funding from?
In July 2021, Standigm secured funding of $10 M from pavilion capital to accelerate its global competitiveness. With this funding, Standigm is ready to make more trade opportunities for its AI-driven drug assets in the global market (source).
In March 2019, Standigm raised US $11.5 million in series B round funding to advance its AI-powered drug pipelines toward license-out. This investment has participants from Kakao Ventures, Atinum Investment, DSC Investment, LB Investment, Wonik Investment Partners, as well as Mirae Asset Venture Investment and Mirae Asset Capital. Kakao Ventures, one of the leading early-stage VCs in Asia, has continued to invest in Standigm since its seed round (Source).
Collaborations
Since July 2019, Standigm and SK Chemicals Co., Ltd. are in an innovation partnership, and from then, they are working hand-in-hand in the drug-discovery process. Recently, this combo announced that they have successfully found a new rheumatoid arthritis indication for an FDA-approved drug and have filed a patent (Source).
The research collaboration is aimed at identifying novel lead compounds and repurposing existing drugs for rheumatoid arthritis and nonalcoholic steatohepatitis, leveraging Standigm’s AI-powered drug discovery platforms: Standigm BEST™, Standigm Insight™, and Standigm ASK™. In this, SK Chemicals has shared their expertise in these diseases and validated the predicted targets and compounds through in vitro and in vivo studies.
Further, in 2020, Standigm Inc. along with SK Holdings C&C, Co., opened their AI-based target identification platform, iCLUE&ASK™, on a trial basis to the public. The platform offers to prioritize protein targets for a query disease and provides the results with evidence through an interactive user interface (source).In 2020, India-based Excelra, a global data science, and data analytics company, announced its collaboration with Standigm Inc. In this collaboration, Excelra will provide its small molecule medicinal chemistry intelligence platform GOSTAR to Standigm Inc. GOSTAR provides comprehensive information encompassing over 8 million compounds, manually curated from 3 million patents and 200,000 journal articles. The database contains over 28 million SAR-associated data points. A well-structured relational database can be utilized for diverse applications across different stages of the drug discovery and development lifecycle and aids in target validation, hit identification, early lead identification, and optimization (source).
In 2017, CrystalGenomics, Inc. and Standigm, Inc., announced their collaboration to apply Artificial Intelligence (AI) for the research and development of novel drugs. In this agreement, both parties plan to work together by combining the power of Standigm’s AI technology with CrystalGenomics’ pharmaceutical expertise to discover and develop novel drugs in the therapeutic area of cancer, rheumatoid arthritis, and liver-related diseases (source).

Digital Healthcare Trends Report 2025
Download The Report3. Genesis Therapeutics
Genesis Therapeutics is a USA-based start-up, founded in 2019. It unifies AI and biotech to accelerate the discovery of new medicines. The company uses neural networks, biophysical simulation, and a scalable computing platform for the design and development of drugs.
Usually, deep learning software just represents molecules like images and classifies them — like, say, this is a cat picture or this is not a cat picture. But, the AI software of genesis therapeutics represents the molecules more naturally. A set of nodes or vertices, atoms, and things that connect them, bonds. They don’t just represent them as a bond or no bond. But as multiple contact types between atoms, spatial distances, and more complex features.
The resulting representation is richer and more complex. A more complete picture of a molecule than you’d get from its chemical formula, or a stick diagram showing the different structures and bonds. Because in the world of biochemistry, nothing is as simple as a diagram. Every molecule exists as a complicated, shifting 3D shape or conformation. Where important aspects like the distance between two carbon formations or bonding sites are subject to many factors. Genesis attempts to model as many of those factors as it can.
Representation is the first step; the next question is, how does one leverage that representation to learn a function that takes an input and outputs a number? Like binding affinity or solubility, or a vector that predicts multiple properties at once?
The startup works at the intersection of modern deep neural network approaches and biophysical simulation — conformational changes in ligands and proteins.
Their Dynamic PotentialNet technology helps in protein structure prediction. It leverages 3D structural information of proteins, and computational protein folding. Also, their AI platform helps in understanding the absorption, distribution, metabolism, elimination, and toxicity (ADMET) properties of drug candidates. (Source)
So far the startup has only one patent. Nevertheless, an interesting one.
US20020046054A1 – This patent majorly focuses on a method for identifying individuals for clinical trials. This method comprises identifying and recruiting donors whose demographic characteristics, genomic and proteomic profile, and medical histories make them attractive candidates for clinical trials, drug target identification, and pharmacogenomic studies.
All in all this method enables efficient identification of research subjects and hence can effectively allow the biopharmaceutical industry to gain access to a large and varied population of individuals with detailed and fully consented medical history/data as subjects for clinical trials required for drug development and as sources of research materials.
Where is the startup receiving its funding from?
In December 2020, Genesis Therapeutics secured a $52M Series A to further accelerate AI innovation and launch a drug discovery & development pipeline. (Rock Springs Capital, T. Rowe Price Associates, Inc., Seed-round lead investor Andreessen Horowitz, Menlo Ventures, and Radical Ventures – source).
In November 2019, Genesis Therapeutics, Unifying AI and Biotech Raised $4.1M in Seed Funding Led by Andreessen Horowitz to Accelerate and Optimize Drug Discovery/Development. (source).
Collaborations
In 2020, Genesis Therapeutics entered into a multi-target collaboration agreement with Genentech, a member of the Roche Group. The collaboration leverages Genesis’ graph machine learning and drug discovery expertise to identify innovative drug candidates for therapeutic targets in multiple disease areas (source).
4. Data2Discovery
USA-based Data2Discovery was founded in 2012. It is applying AI to find hidden connections and new insights in diverse, linked datasets. Their healthcare data analytics platform is intended to get results from large amounts of complex heterogeneous data. The company’s platform uses an advanced stack of scalable graph technologies, public and proprietary data sources, AI and machine learning, graph mining capabilities, and extensive experience in linking and mapping data to address problems, enabling users to get results efficiently and effectively.
This startup has only one patent, US20190130290A1. This patent discloses a method for semantic analysis of disparate (different or diverse) data in an environment having a plurality of datasets with distinct information fields.
Further, this method involves creating graphs relating to specified information with information fields from multiple datasets as nodes with improved accuracy of machine learning in digital computers.
This technology is basically a data-driven process. There are vast data available related to drug discovery processes which include exploration data of different chemical moieties, clinical trial data of different drugs, early-stage chemical molecules, and their effectiveness data, etc.
All this data comprises some effective drug molecules which can be further explored and can become a potential drug substance for treating any condition. Data2Discovery is utilizing its platform and software to explore and screen all the available data related to the specific drug discovery processes and identify the required molecule or the step where researchers need to spend more time to get the required chemical moiety. Their platform uses all this data along with AI/ML technology to plot different scalable graphs, graph mining, and linking & mapping data to address the problems to provide the required results efficiently and effectively.
Where is the startup receiving its funding from?
In March 2017, Data2Discovery was awarded a $750,000 grant from the National Science Foundation (NSF) via the highly competitive Small Business Innovation Research (SBIR) Phase II program.
The grant will support Data2Discovery’s efforts to support translational and phenotypic research on vast interlinked datasets; which will include applications in Drug Repurposing, Toxicology and Safety, and Phenotypic Analysis (source).
Collaborations
In April 2017, the Open PHACTS Foundation announced the collaboration with Data2Discovery to form a Strategic Partnership. The goals of the partnership will support and continue with the Open PHACTS vision of creating a sustainable, open, interoperable information infrastructure for applied life science research and development while advancing science for the public benefit through shared knowledge and data in life science and biomedical research.
Data2Discovery Inc claims to bring extensive experience in pharmaceutical semantic linked data. The startup partners with pharmaceutical companies to develop full-stack semantic and graph capabilities to venture into real scientific problems (source).
In February 2017, Data2Discovery Inc identified 14 potential drug repurposing opportunities for Tuberculosis (TB) by applying its P3 graph-based association finding approach. This is done in an innovative partnership with the NIH National Center for Advancing Translational Sciences (NCATS), and OpenPHACTS. This small-scale project successfully demonstrated the feasibility of combining two key data resources – EU OpenPHACTS Open Pharmacological Space (OPS) and NCATS Phenotypic Drug Discovery Resource (PDDR) – with state-of-art graph mining tools from Data2Discovery. The capabilities demonstrated in this project open up many opportunities for public impact in rare and neglected diseases, as well as complex disease areas being pursued by pharmaceutical companies (source).
In May 2021, Data2Discovery along with Indiana University Crisis Technologies Innovation Lab and two partner companies Disaster Tech and OPS was awarded a $2.3m contract from the US Army Telemedicine and Advanced Technology Research Center (TATRC) to create a Technology in Disaster Environments Learning Accelerator (TLA). The TLA will employ advanced data and performance science tools to identify best practices for patient care in disaster and infrastructure-degraded environments as part of the National Emergency Tele-Critical Care Network (NETCCN). Data2Discovery will use its proprietary graph technology stack along with deep expertise working with medical and biomedical data to pilot capabilities that will permit insights to be gained from multiple data streams that cannot be found elsewhere (source).
5. Unlearn.AI
Founded in 2017, Unlearn.AI is a platform designed to make computational clinical trials. The company’s platform accelerates clinical trials by supplementing control groups with synthetic patient data generated using AI, which helps in reducing the time to develop new medicines, enabling healthcare companies to sooner provide patients in need with life-saving therapies.
Technology – Unlearn is the only company using AI to create Digital Twins, which helps in accelerating clinical trials and getting better results. Unlearn’s platform utilizes historical datasets and disease-specific machine-learning models to generate virtual placebo patients, created from actual patient baseline data in clinical studies. This novel approach increases trial power and confidence, accelerates trial timelines, and enables patient-level insights. The whole process involves the following steps –
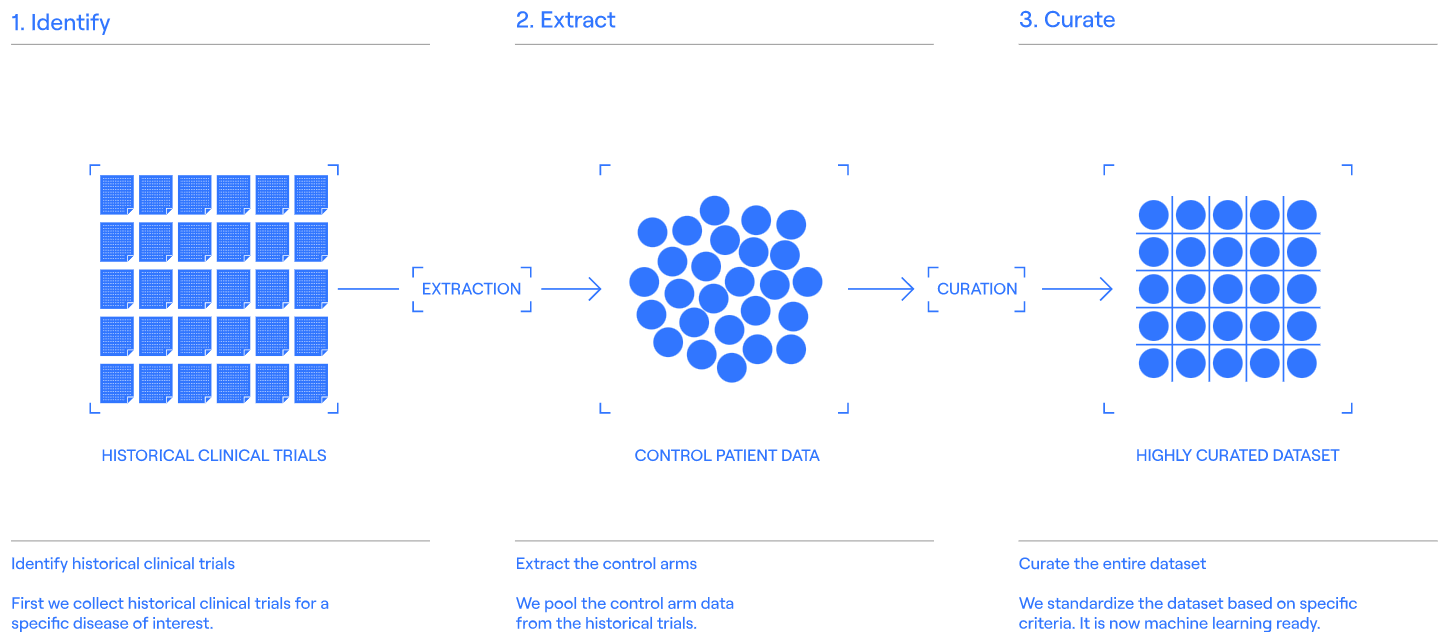
Step 01 [Creating a dataset]
The first step involves the preparation of a highly curated dataset, so the machine learning model can learn from the relationships.
Step 02 [digenesis-generating-our-machine-learning-model]
After preparing the dataset, it needs to be separated into two groups, one for training and the other for testing. The machine learning model builds an internal network of connections and starts generating Digital Twins.
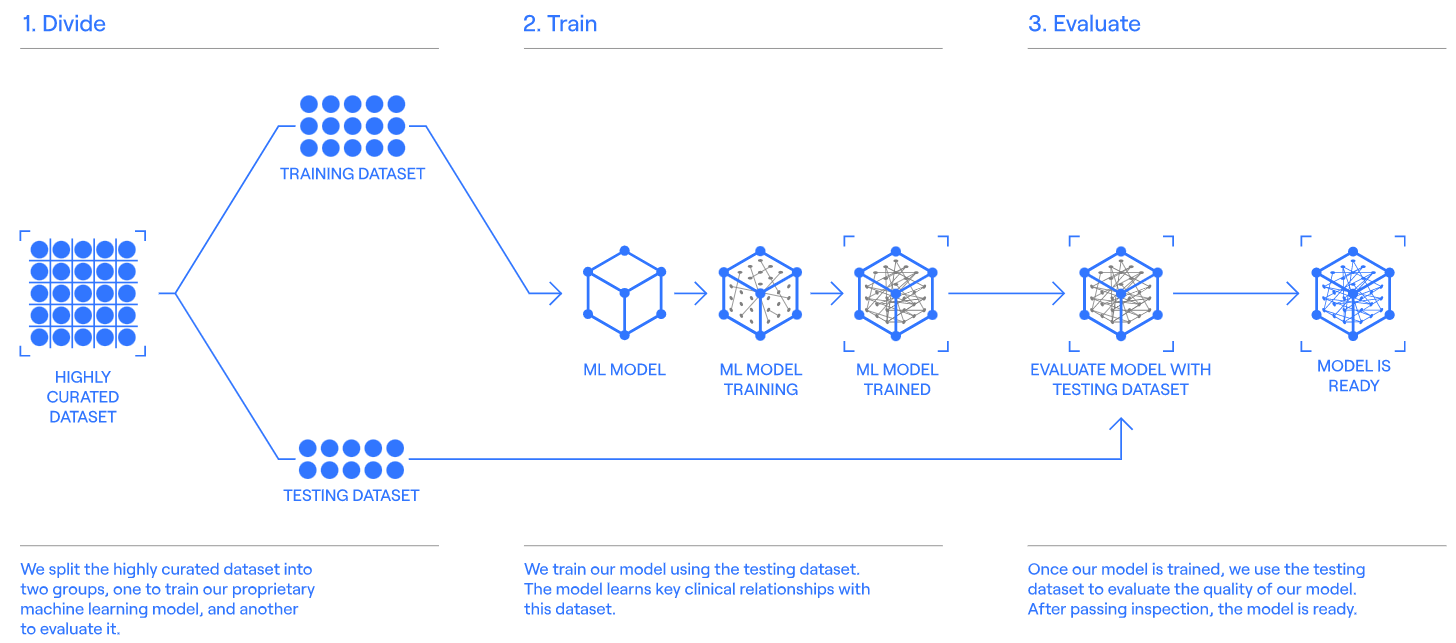
Step 03 [digital-twin-procova]
Once the trial has started, the platform makes records of the Digital Twin of patients. This model uses baseline data to create a complete record that predicts how the patient would have responded if he/she had not received the experimental treatment. Next, the use of PROCOVA™ (prognostic covariate adjustment) – a statistical method that incorporates Digital Twins into statistical analysis plans to provide a more precise estimate of the treatment effect.
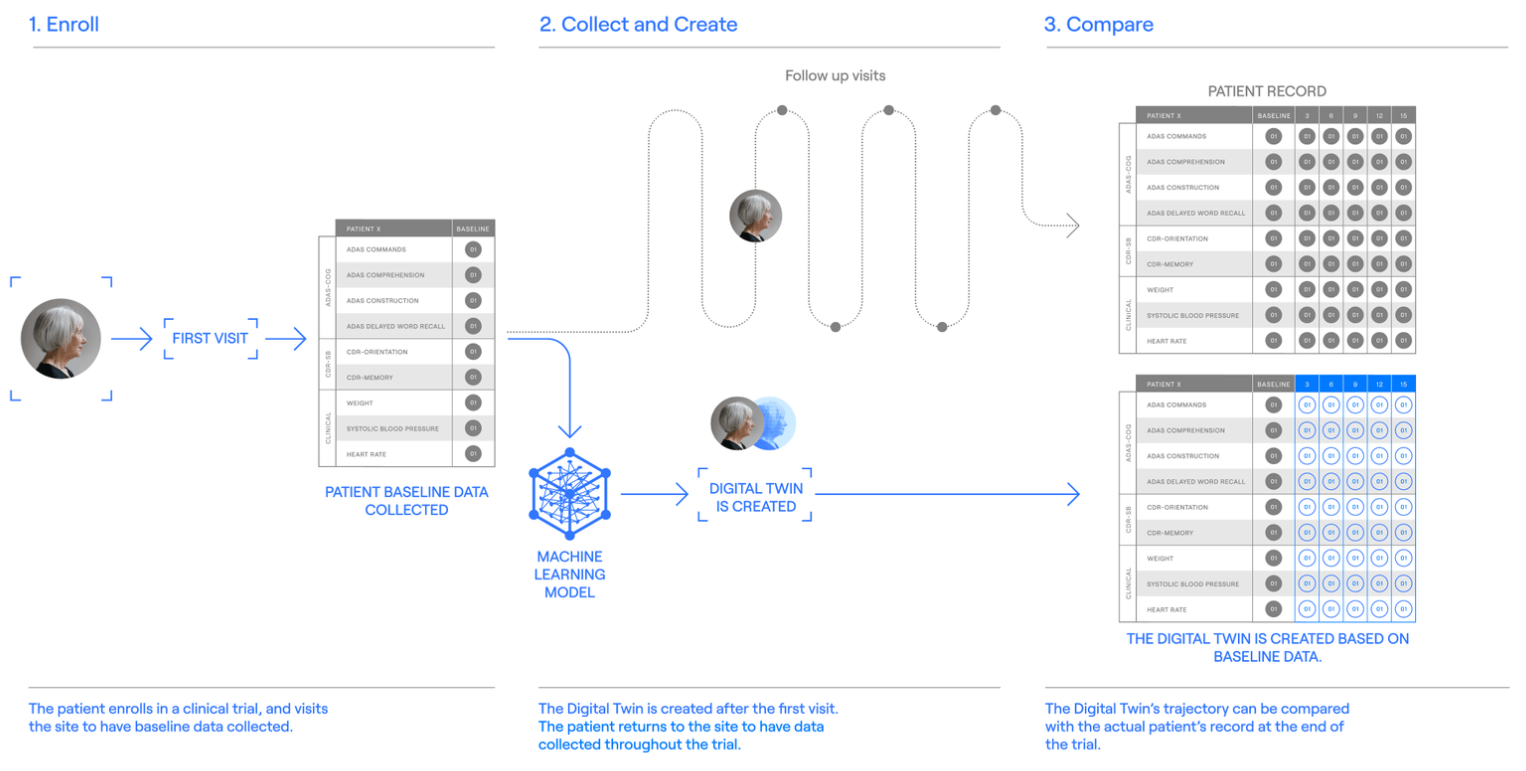
Step 04 [Randomized controlled trials using Digital Twins and PROCOVA™]
The prepared digital twins are incorporated into clinical trials, which helps in enabling smaller, more efficient trials. Each patient from the trial was paired with their AI-generated predicted placebo outcome or with Digital Twin. Digital Twins maintain randomization and blinding, and increase certainty without introducing bias.
The startup has filed three patent applications. Let’s discuss each of them in brief.
- CA3088204A1 – This patent describes a method to train an artificial intelligence system or an artificial neural network that can provide a probability of results based on provided inputs/data. This trained AI model probability identifier can be utilized in various fields such as health informatics, image/audio processing, marketing, sociology, and lab research fields.
- WO2021041128A1 – This patent discloses a method for determining the treatment effects of randomized control trials (RCT). The method includes steps for receiving data from an RCT, generating results using different models (e.g., Conditional Restricted Boltzmann Machine, a recurrent neural network, etc.), and determining treatment effects for the RCT using the generated results. This method estimates quantities with high accuracy and precision and determines decision rules for declaring treatments to be effective that have low error rates.
- US20210117842A1 – This patent describes a method to train generative models (a machine learning model that learns to sample with the observed data) using summary statistics, so that data generated by the model satisfy specified, population-level summary statistics. Further, these generative models are utilized in a variety of fields, such as economic forecasting, climate modeling, and medical research.
All these patents explain technologies that can be used in speeding up the highly time-consuming process of clinical trials. Unlearn’s patents disclose the use of machine learning or artificial neural networks such as the restricted Boltzmann machine (RBM), a recurrent neural network, etc. in the clinical trial process and its data to get effective results.
Further, their technology also uses machine learning to create Digital Twins, which helps in accelerating clinical trials and checking the effect of particular drugs before the actual testing on humans. This technology is a breakthrough in drug discovery as it can simplify the whole clinical trial process and will provide its outcome early and accurately. Performing clinical trials using such an advanced solution will definitely reduce the time and money spent in the drug discovery process.
Also, for their breakthrough innovation using AI, Unlearn.AI won the “Predictive Analytics Solution of the Year” in the 2021 BioTech Breakthrough Awards Program (Source).
Where is the startup receiving its funding from?
In Nov. 2020, Unlearn.AI announced a series of extensions with new investments from Epic Ventures, alumni ventures groups, and global pharma company Eisai (source).
In Apr. 2020, Unlearn.AI closed a $12m series to advance the use of digital twins in clinical trials. Led by 8VC, this financing will accelerate the application of Unlearn’s innovative machine-learning technology to improve clinical trial efficiency and increase confidence in results (source).
These were the 5 AI-based startups that are making ripples in Drug research. But there are more startups bringing in other technologies than AI in their drug research market.
6. Recursion
Founded in 2013, Recursion Pharmaceuticals is a leading drug discovery startup that merges experimental biology, bioinformatics, and artificial intelligence to pinpoint treatments for cellular-level diseases. They aim to accelerate drug discovery by combining biological insights with cutting-edge machine learning, targeting rare and common diseases.
Based in Salt Lake City, Utah, Recursion has crafted an AI-powered platform to speed up the identification of therapeutic candidates. Notably, they’ve already progressed a candidate into Phase 1 clinical development for a rare hereditary stroke syndrome using machine learning, a remarkable departure from the traditional drug development timeline.
Collaborations
- On July 12, 2023, recursion announced a collaboration and a $50 million investment from NVIDIA to accelerate foundation models in Ai-enabled drug discovery. The companies collaborated on software for biotech and pharmaceutical companies to create improved patient treatments faster.
- In December 2021, the startup initiated a multi-year collaboration with Roche and Genentech, focusing on neuroscience and oncology. This partnership utilizes Recursion’s OS and Biology Maps and Roche and Genentech’s single-cell perturbation screening data to uncover new biological connections for innovative therapeutic initiatives swiftly. The alliance aims to launch potentially 40 programs over a decade or more.
- In August 2020, Recursion formed a strategic, multi-year partnership with Bayer focused on fibrosis. This was extended in December 2021 to incorporate their advanced inferential search capabilities. In this enhanced collaboration, the companies are working on over a dozen initiatives to discover new therapies for challenging fibrotic conditions affecting various organs like the lungs, liver, and heart.
Acquisitions
On May 8, 2023, Recursion entered into agreements to acquire Cyclica (Decentralized Drug Discovery) for $40 million and Valence (Drug Design Company) for $47.5 million.
Fundings
Recursion Pharmaceuticals secured a total of $665.4 million through 18 funding rounds. Their most recent funding was obtained via a Post-IPO Equity round on July 12, 2023.
7. BenevolentAI
Headquartered in London, with research centers in Cambridge (UK) and New York, BenevolentAI was founded in 2013 with the vision of leveraging AI to revolutionize scientific discovery.
BenevolentAI is a prominent player in AI-driven drug discovery and development, traded on the Euronext Amsterdam stock exchange. By combining its advanced AI platform, scientific acumen, and lab capabilities, the company aims to deliver innovative drug candidates with a higher likelihood of clinical success compared to traditional approaches.
The startup has a growing portfolio of 13 named drug programs and over 10 exploratory initiatives, powered by the Benevolent Platform™.
Collaborations
- In 2016, BenevolentAI secured an exclusive license for a collection of clinical-stage drug candidates from Janssen Pharmaceutica, a subsidiary of the Janssen Pharmaceutical Companies under Johnson & Johnson.
- In 2019, BenevolentAI collaborated with AstraZeneca to use AI and machine learning to develop new therapies for chronic kidney disease (CKD) andidiopathic pulmonary fibrosis (IPF).
- Further in 2022, BenevolentAI and AstraZeneca decided to extend their collaborative AI-driven drug discovery initiative. This expansion encompassed systemic lupus erythematosus (SLE) and heart failure (HF) in addition to previous disease areas. By harnessing the capabilities of the Benevolent Platform and biomedical Knowledge Graph, scientists and technologists from both organizations stand to gain a deeper insight into the fundamental disease mechanisms and uncover novel targets for therapeutic intervention.
- In 2023, 9xchange joined forces with BenevolentAI to integrate its Ai technology into its innovative Biopharma Marketplace. This collaboration will help promote information sharing and collaboration across the biopharma industry to accelerate new drug discovery and development.
Acquisitions
In December 2021, BenevolentAI agreed to merge with Odyssey, a special-purpose acquisition company listed on Euronext Amsterdam with a valuation of €300 million. Odyssey fosters growth within European healthcare and technology, media, and telecommunications (TMT) companies.
Fundings
BenevolentAI secured a total of $292 million through three funding rounds. Their most recent funding was acquired through a Private Equity round on September 17, 2019.
8. Xbiome
Xbiome stands as China’s first AI pharmaceutical firm dedicated to intestinal micro-ecology. With a foundation in this field, it employs AI technology to precisely and individually oversee the gut health of the nation’s 1.3 billion individuals. Employing artificial intelligence, Xbiome analyzes both patients’ and donors’ intestinal flora to expedite the development of impactful pharmaceutical solutions.
Collaborations
In January 2022, Aurealis Therapeutics, a synthetic biology company developing groundbreaking four-in-one cell and gene therapies and Xbiome, announced that the two companies have entered into an exclusive license and collaboration agreement to advance the clinical development and commercialization of Aurealis’ investigational Diabetic Foot Ulcer (DFU) therapy, AUP-16, as well as its application for other chronic wounds and inflammatory diseases in Greater China.
As per the terms of the agreement, Xbiome secured exclusive rights for the development and commercialization of AUP-16 in the clinical stage, specifically for Diabetic Foot Ulcer, within Mainland China, Hong Kong, Macao, and Taiwan. Xbiome undertook clinical development, regulatory submissions, and commercialization of these licensed products across the designated territories.
Acquisitions
During April 2022, Xbiome revealed its acquisition of the clinical-stage M201 program from Assembly Biosciences, Inc. This biotechnology company is known for its focus on developing innovative therapeutics that target hepatitis B virus and other viral diseases.
Fundings
Xbiome secured a sum of $124.2 million through 4 rounds of funding. Their most recent financing was obtained during a Series B round on December 22, 2021.
9. Insilico Medicine
Insilico Medicine provides an AI platform for developing drugs for cancer and age-related diseases. Their all-encompassing pipeline covers drug discovery, clinical trial analysis, and digital medicine.
With active involvement in internal drug discovery ventures, Insilico addresses conditions like cancer, dermatological diseases, fibrosis, Parkinson’s Disease, Alzheimer’s Disease, ALS, diabetes, sarcopenia, and aging.
This startup takes the lead as the first fully generative AI drug to enter human trials. The company uses generative AI to speed up rare disease drug discovery and aims for treatments targeting idiopathic pulmonary fibrosis, Covid-19, and cancer.
Their AI platform identifies drug candidates, anticipates clinical trial outcomes, and associates molecules with target compounds. Contrasting traditional methods that consume six years and over $400 million, Insilico achieves this at one-tenth of the cost and one-third of the time, progressing to Phase II trials in merely two and a half years. (Source)
Collaborations
- In July 2023, the startup partnered with MIKADO to discover new cystinosis treatments. The Mechanisms of Inherited Kidney Disorders (MIKADO) team from the University of Zurich employed Insilico Medicine’s AI target discovery engine, PandaOmics. This AI engine identified and validated actionable drug targets for cystinosis, a lysosomal storage ailment, in preclinical models.
- In November 2022, Insilico Medicine entered into a significant research partnership with Sanofi valued at a potential $1.2 billion. According to the arrangement’s conditions, this collaboration utilized Insilico Medicine’s AI platform, Pharma.AI, to progress drug development prospects for as many as six novel targets.
- In March 2022, Insilico Medicine and EQRx joined forces for AI-driven drug discovery, development, and commercialization across several targets. This collaboration utilizes Insilico’s Pharma.AI platform for finding new small-molecule drug candidates, while EQRx’s unique approach speeds up drug development and affordable patient access to innovative medicines.
- In January 2022, Fosun Pharma and Insilico Medicine revealed an AI-driven collaboration for global drug discovery and development. This partnership aims to utilize AI technology to advance drugs for various targets.
- In September 2021, Huadong Medicine and Insilico Medicine initiated a co-development alliance to push forward oncology drug discovery, explicitly focusing on hitting undruggable targets.
Fundings
Insilico Medicine secured a funding of $401.3 million across ten funding rounds. Their most recent funding was through a Series D round on August 10, 2022.
10. Xilis
Founded in 2019, this biotech firm is dedicated to advancing precision oncology through a unique platform. Xilis’s platform assists oncologists in making informed treatment choices to enhance patient outcomes in cancer care. Additionally, it aids pharmaceutical companies in their drug discovery and development endeavors.
At the core of their approach lies the utilization of patient biopsies to cultivate miniature replicas of their distinct organs and tumors. Medical professionals analyze these tissue samples to identify the disease stage and assess its reactions to diverse drugs and therapies. This enables swift determination of the optimal treatment path.
Collaborations
In February 2023, the University of Texas MD Anderson Cancer Center formed a strategic partnership with biotech firm Xilis to accelerate the progress of cutting-edge technology and expedite the development of novel cancer therapies.
This collaboration involves the integration of Xilis’ MicroOrganoSphere (MOS) platform with MD Anderson’s specialized knowledge, synergizing efforts to advance therapy development and cancer research.
Within this agreement, the joint focus of MD Anderson and Xilis is to propel drug development and research initiatives forward using the innovative MOS technology.
Fundings
Xilis has secured funding of $92 million across five funding rounds. Their most recent financing was obtained through a Series A round on July 13, 2022.
AI and ML Startups you can acquire in Drug Discovery
1. Atavistik Bio
Founded in 2021, Atavistik Bio emerges as a recently established, privately-held biopharmaceutical entity. The company is dedicated to advancing novel therapeutic solutions for metabolic diseases and cancer.
Their innovative strategy involves screening libraries of metabolites and proteins to identify important binding sites. This approach empowers them to create and advance transformative therapies. Their efforts primarily focus on rectifying protein functions to address innate metabolic errors.
Additionally, they employ metabolites to enhance the development of cancer treatments. Their technology and analytics platforms further allow them to expand their reach into different disease domains.
Collaborations
In January 2023, Atavistik Bio announced a collaboration with Plex Research to enhance its AMPS Platform’s informatics capabilities. This collaboration aims to speed up the identification of innovative small-molecule therapeutics.
Fundings
In August 2021, the startup secured a Series A financing of $60 million. The funds acquired were dedicated to progressing genetically-validated targets in metabolic diseases and cancer.
2. Polaris Quantum Biotech
Established in 2020, Polaris Quantum Biotech is based in Durham, North Carolina. Polaris Quantum Biotech is transforming drug design by integrating quantum computing, artificial intelligence, and precision medicine. By merging these technologies, the company can navigate extensive chemical libraries, pinpointing potential drugs that specifically target proteins for precise disease modulation.
Collaborations
During February 2022, Allosteric Bioscience, a company that enhances treatments for Aging and Longevity by combining Quantum Computing, Artificial Intelligence, and Biomedical sciences, entered into a collaboration agreement with Polaris Quantum Biotech. Together, they are harnessing the progress in Quantum Computing and Artificial Intelligence to innovate in the domain of pharmaceutical development.
Fundings
Polaris Quantum Biotech secured $2.1 million in funding through a single Seed round, which took place on August 10, 2021.
3. AMPLY Discovery
AMPLY Discovery is an innovative enterprise specializing in crafting novel biogenic molecules with potential anti-infective applications for both animal and human health contexts.
Through the utilization of advanced machine learning techniques and synthetic biology methodologies, AMPLY Discovery delves into expansive biological datasets to find novel candidates for drugs and nutraceuticals. Their proprietary in silico and in vitro hybrid platform facilitates this process, allowing them to identify superior molecular entities.
Their pursuits extend to addressing significant challenges such as cancer, multi-drug resistant infections, and metabolic disorders.
Fundings
AMPLY Discovery has secured a total of £900,000 in funding across 2 rounds. They acquired their most recent funding on September 21, 2022, through a Seed round.
What other technologies are companies bringing into drug research?
Apart from these technological advancements, other companies are also using different technologies in speeding up the drug discovery process. For example, an Australian-German start-up, Quantum Brilliance is using ultra-efficient quantum computers to reveal previously unknown compounds and uses quantum accelerators in the drug discovery process. Big pharma companies such as Boehringer Ingelheim, and Merck are collaborating with this start-up to expedite the drug discovery process (source).
Also, Secondcell Bio and Alliance Care Technologies International announced a new strategic partnership to use Chromovert® Technology in the rapid drug discovery process to combat rare genetic diseases. This technology uses a cell-based discovery platform to screen the cells and choose the best cell which gives effective responses to the molecules which are in the drug discovery process (source).
Future Outlook
Artificial intelligence (AI) or Machine Learning (ML) is an advanced technology that is going to hold the future in its hands. Using these technologies in the drug-discovery process will help in quickly identifying new targets and providing the cure for incurable diseases.
With the predicted market growth estimation and the increased number of working companies and startups in this domain, we can confidently state that the future of drug discovery appears extremely promising. But the market is still new and for drug giants to have an edge in the industry. It is important they figure out startups that align with their goals and collaborate with them.
Learn how GreyB can help you find your desired startups and scale-ups, or reach out to us directly:
How Can We Help You?
We support industry-leading R&D and Innovation professionals through complex problems. Describe your challenge, and let us bring clarity and expertise.
Authored By: Ganesh Solanke and Nikhil Gupta, Search Team.