Value Delivered
The investigation enabled the client to uncover a hidden third-party patent that indirectly covered Denosumab formulations by referencing the COM patent. Identifying this risk early protected substantial investment and prevented potential litigation delays in a market valued at over $3 billion annually. The findings provided clear strategic boundaries for formulation design and helped the client’s R&D team make informed decisions.
Problems Solved
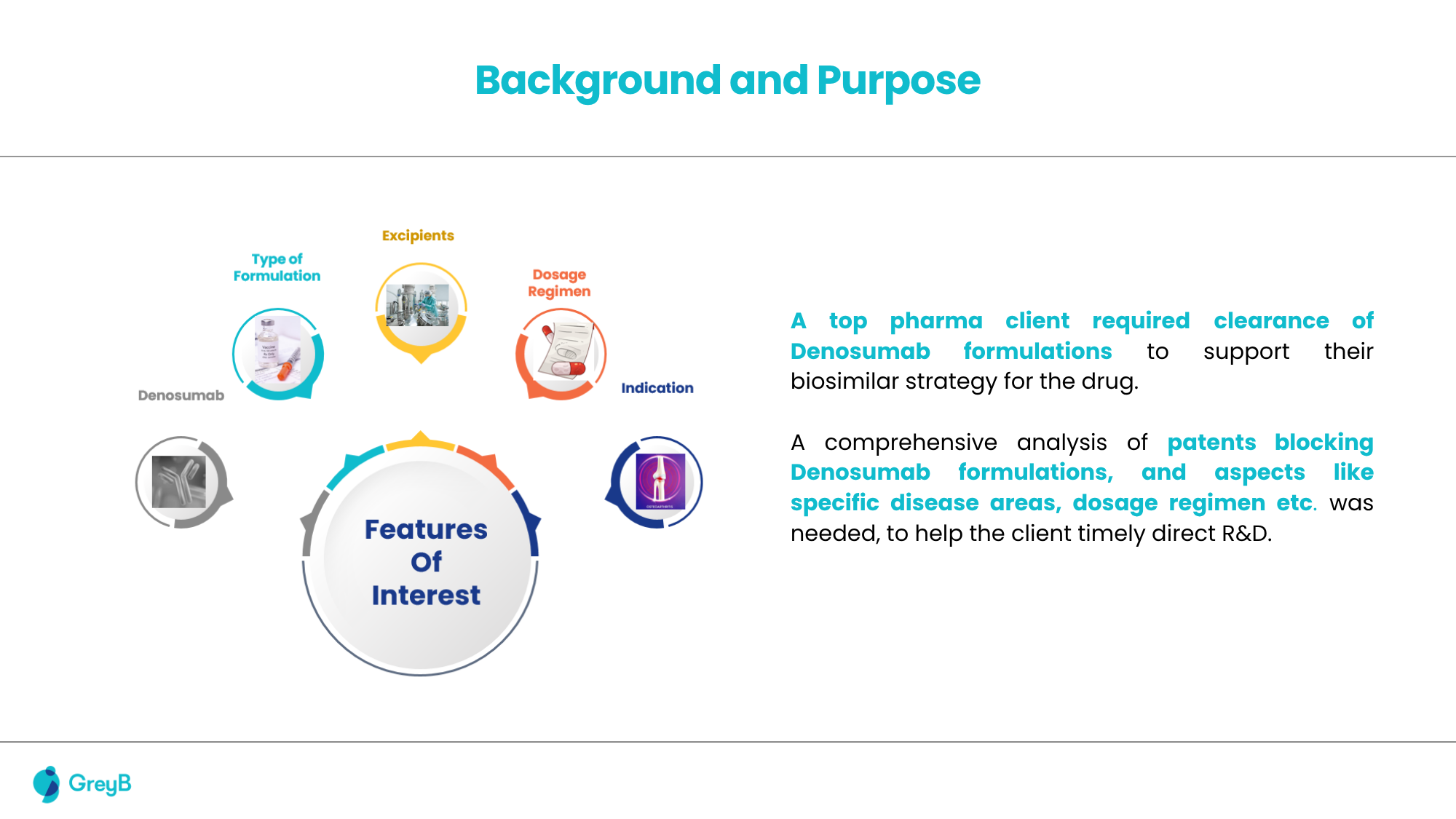
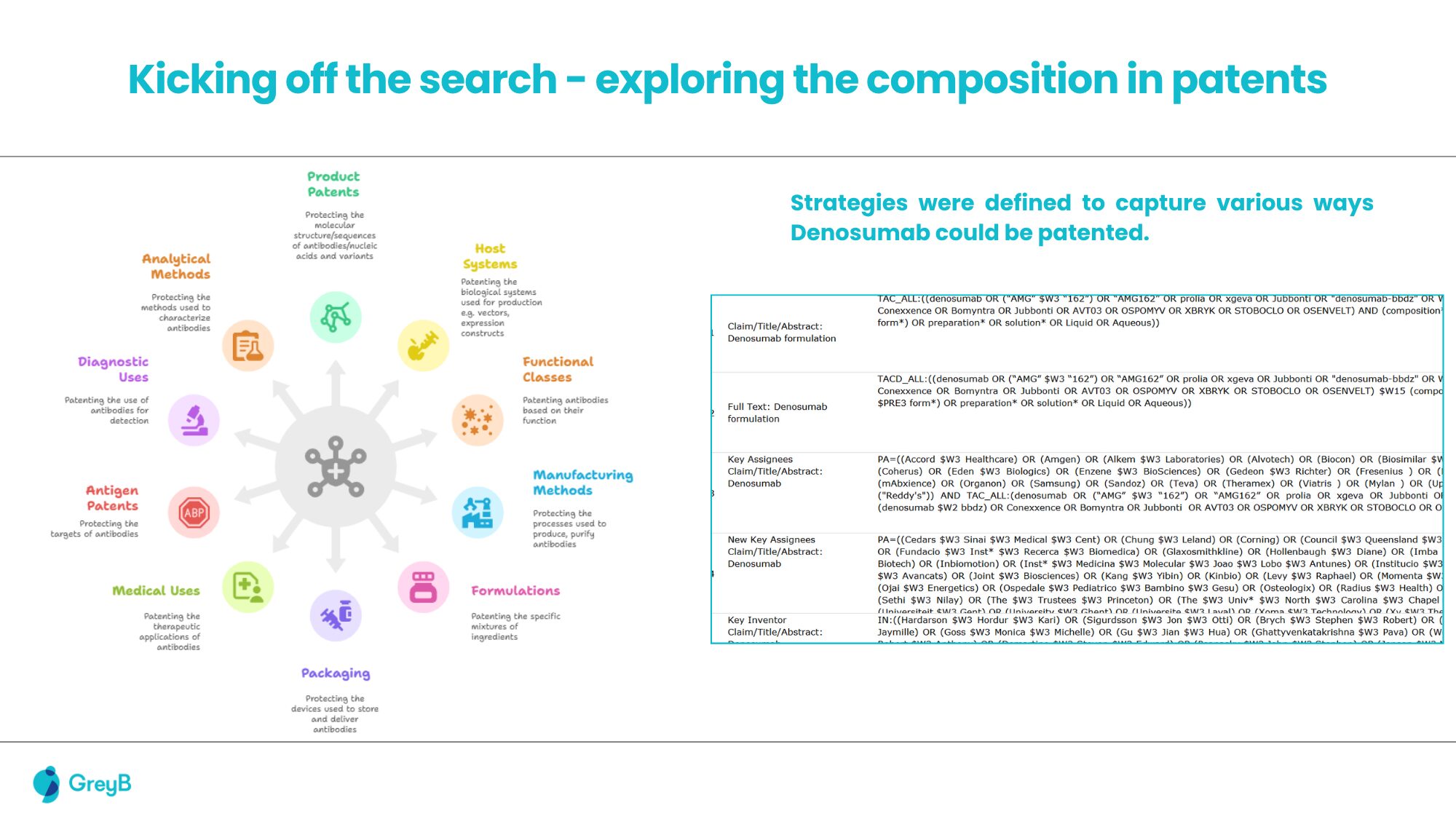
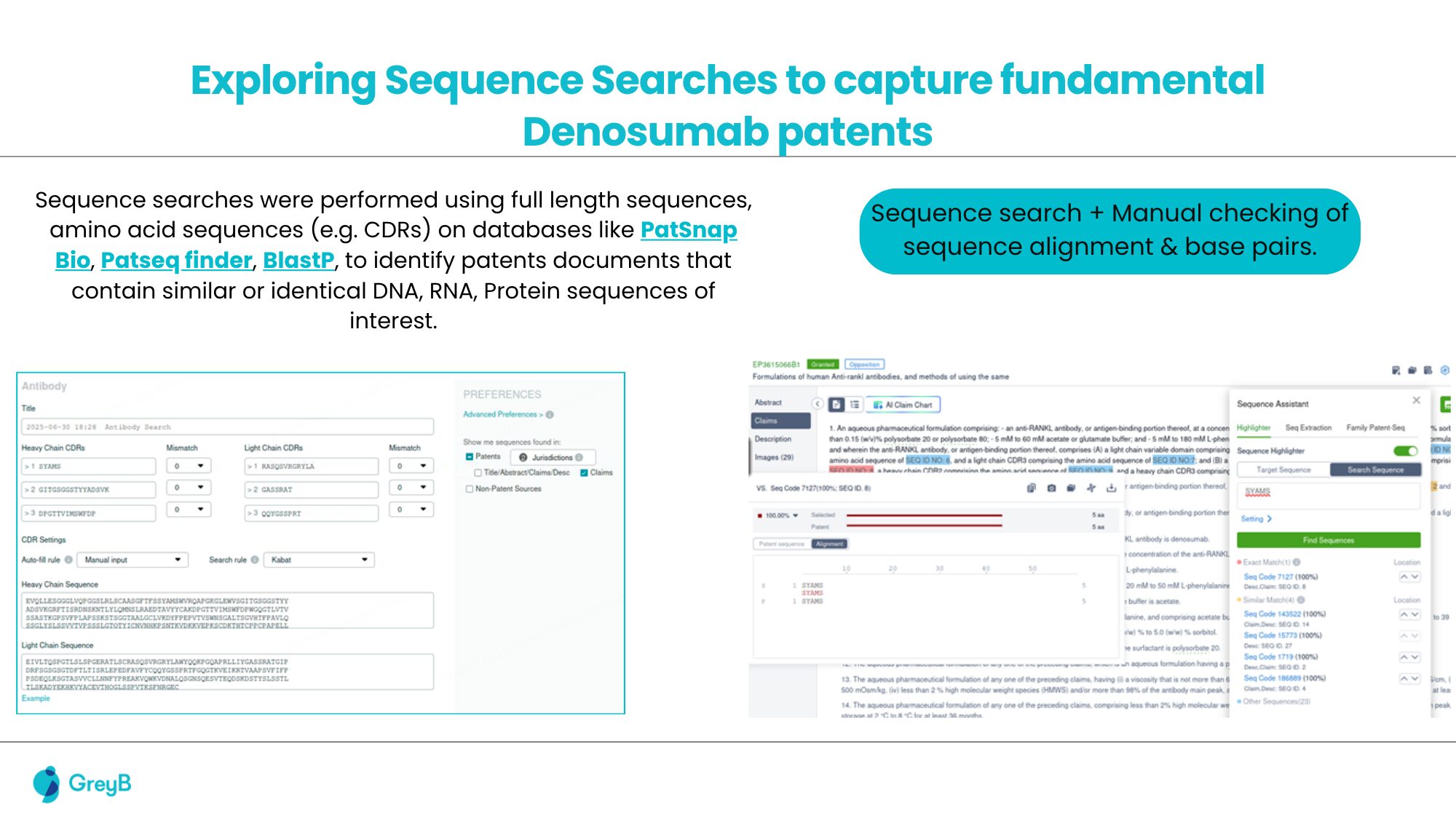
A leading biologics developer preparing to commercialize a Denosumab-based therapy faced a significant challenge typical in antibody development: navigating a dense patent landscape. Standard Freedom-to-Operate (FTO), including Purple Book analysis and sequence-based searches on platforms such as Patsnap Bio, was initially performed.
These efforts identified several publications directly claiming denosumab antibodies, and functional class-based, composition-of-matter (COM) patents like (WO 03/002713) covering Denosumab’s sequence. However, these conventional methods failed to detect indirect blocking patents, particularly those that did not mention Denosumab or its sequence yet restricted its manufacture or formulation.
Missing out on such publications would have exposed the company to potential infringement risks and late-stage setbacks in its R&D cycle.
Solution Offered
A comprehensive, multi-layered FTO investigation was conducted. Deeper analysis focused on patents that directly referenced this COM patent within their specifications. This search uncovered a formulation patent broadly claiming liquid protein compositions containing specific excipients.
Although the patent did not claim Denosumab, its sequence, or functional class, the explicit reference to the COM patent within the specification indicated a high potential for infringement of the client’s Denosumab formulation and was flagged to the client.
Read the full case study to see how GreyB’s patent intelligence protected a pharmaceutical company from a potential infringement dispute in a $3 billion market.
Request Full Case Study
Download in PDF Format and read anytime.