Over 6,300 pharma startups are currently active worldwide, collectively pushing the boundaries of drug development with AI-powered platforms that can reduce discovery timelines from years to months and slash costs by up to 70%.
While traditional drug development typically takes 10-15 years and costs exceed $2 billion, a new generation of companies is leveraging artificial intelligence, gene editing, enzymatic synthesis, and programmable cell therapies to compress these timelines dramatically.
AI healthcare startups alone raised $10.5 billion across 511 deals in 2024, with landmark investments like Xaira Therapeutics’ $1 billion Series A signaling unprecedented confidence in computational drug discovery.
Yet despite this funding boom and technological promise, a critical question remains: which specific startups are truly positioned to translate platform innovation into clinical breakthroughs?
The following 10 startups represent the most compelling answers, each tackling fundamental bottlenecks across target discovery, manufacturing scalability, precision diagnostics, and therapeutic control.
1. Insilico Medicine
Insilico Medicine addresses some of the most complex and underserved diseases, beginning with idiopathic pulmonary fibrosis (IPF), a progressive and fatal lung condition with limited therapeutic options.
The company’s core product is Pharma.AI, an end-to-end generative AI drug-discovery platform that integrates Biology42 for target discovery, Chemistry42 for de novo molecule generation, and Medicine42 for clinical-stage optimization.
This AI-driven workflow automates the entire early R&D cycle by:
- identifying novel targets
- generating new chemical matter
- predicting pharmacokinetics and toxicity
- and refining candidates
Thus, significantly reduces the cost and time of discovery compared to traditional methods.
Its lead candidate, ISM001-055 (Rentosertib), is one of the world’s first AI-discovered and AI-designed drugs to reach mid-stage human trials. Designed by Chemistry42 and targeting a fibrosis-related TNIK signaling pathway identified by Biology42, Rentosertib is a first-in-class small molecule intended to halt IPF progression.
Insilico has advanced this molecule through Phase I and Phase II trials, demonstrating a strong safety profile and early clinical signals, including meaningful improvements in lung function compared to placebo. The company publicly shares its efficacy data through scientific posters available on its website, reflecting a commitment to transparency and scientific rigor.
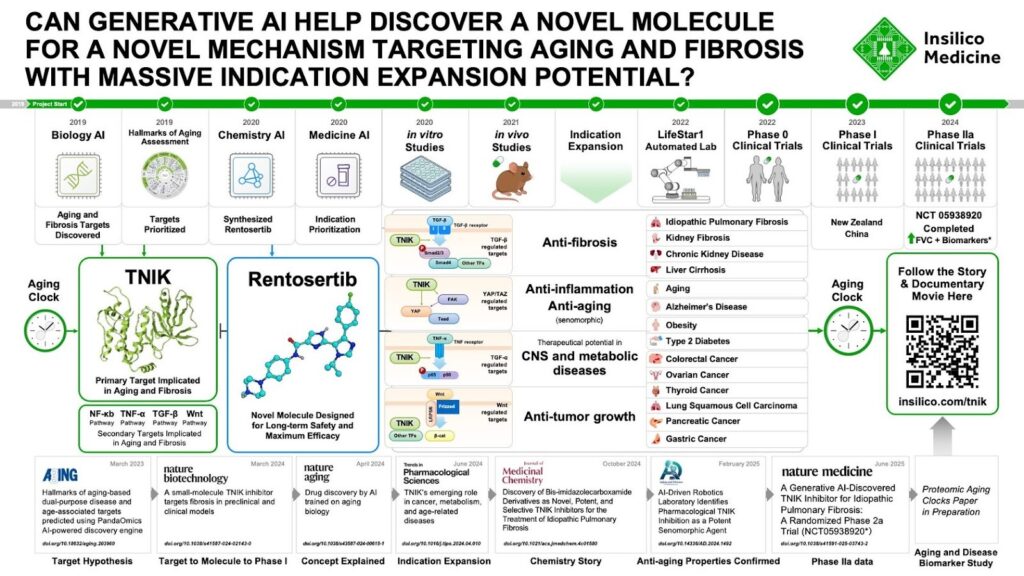
Beyond Rentosertib, Insilico has assembled a growing pipeline of 20+ AI-designed preclinical programs, with more than 10 candidates already in clinical development.
The pipeline spans oncology, such as its MAT2A inhibitor, chronic inflammation, kidney disease, and antiviral therapeutics. These programs are publicly listed on the company’s pipeline portal, where Insilico details each asset’s stage, indication, and AI-based discovery approach.
The commercial impact of Insilico’s platform is significant. Its AI-driven discovery capabilities have attracted multimillion-dollar partnerships with global pharmaceutical companies, including Sanofi, Fosun Pharma, and Exelixis.
These collaborations validate the scalability of its platform and reinforce the shift toward platform-driven R&D across the industry. By reducing early discovery timelines from years to months and cutting associated costs by up to 70%, Insilico is establishing itself as one of the most influential companies pushing AI-discovered drugs toward clinical and commercial reality.
2. Owkin
Owkin focuses on some of the most complex challenges in precision medicine, especially in oncology, where identifying which patients will benefit from specific therapies remains a major obstacle.
The company addresses this by using federated learning, a privacy-preserving AI framework that trains models on sensitive hospital data without moving the data outside the hospital.
This allows Owkin to securely integrate clinical records, whole-slide pathology images, molecular profiles, and real-world outcomes to discover new biomarkers and build highly accurate prediction models.
Owkin Zero
OwkinZero is a specialized AI model developed to accelerate biological discovery, particularly in drug development. Built on over 300,000 curated biomedical Q&A pairs across eight critical domains, it leverages proprietary patient data, spatial and bulk transcriptomics, and extensive domain expertise.
The model is trained using reinforcement learning on benchmark datasets that reflect key challenges, such as target druggability, modality suitability, and drug perturbation effects.
This approach enables OwkinZero to outperform even much larger commercial AI models on specialized tasks, providing clear advantages in both accuracy and reasoning fidelity.
MSIntuit CRC
MSIntuit CRC, its flagship diagnostic, is a CE-marked AI tool that detects microsatellite instability (MSI) directly from H&E histology slides in colorectal cancer.
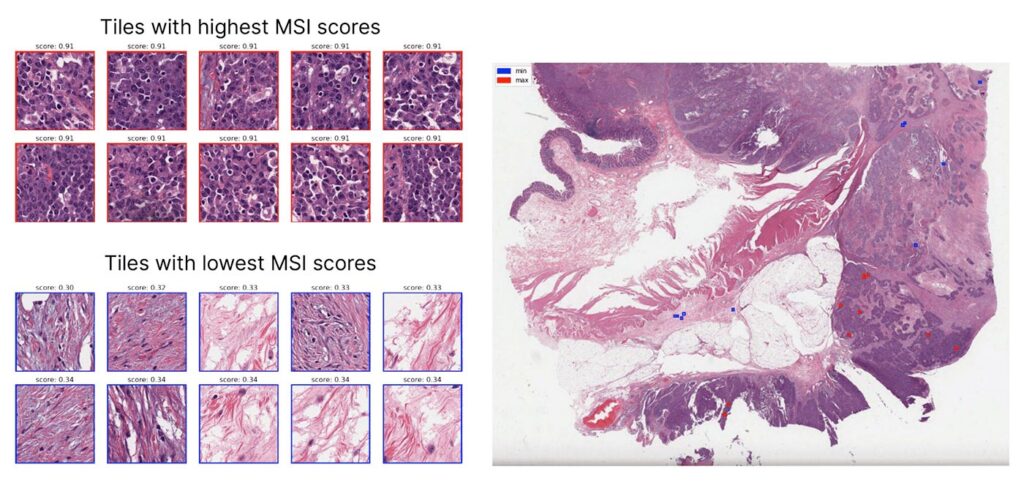
This makes MSI testing faster, more accessible, and significantly more scalable than genomic sequencing, particularly valuable in regions with limited molecular testing capacity.
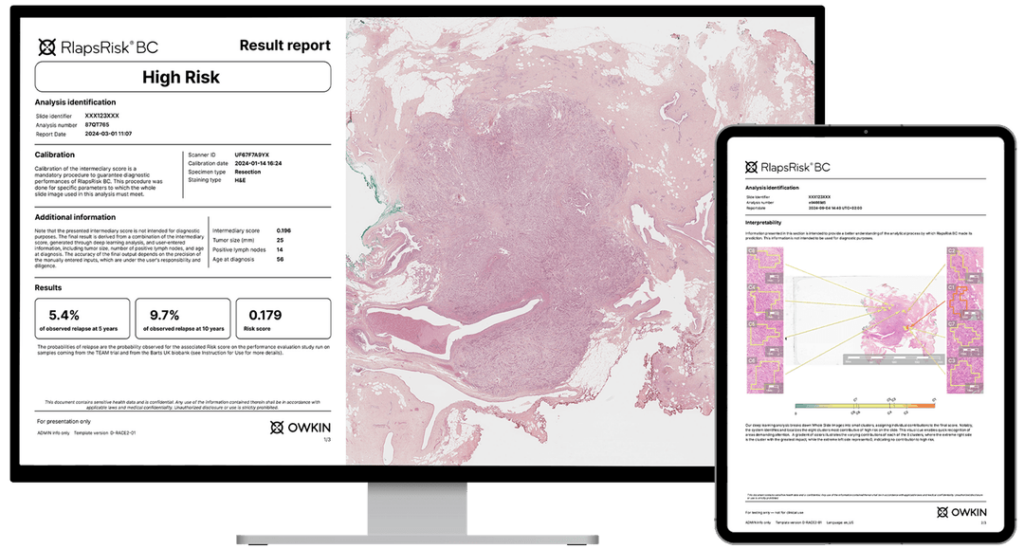
RlapsRisk BC
The company also developed RlapsRisk BC, an AI-powered prognostic tool that predicts relapse risk in early-stage breast cancer by analyzing digitized pathology slides.
The model identifies subtle morphological features that correlate with long-term recurrence, insights often undetectable to human pathologists alone. This tool supports clinicians in refining treatment decisions and avoiding both over- and under-treatment of early-stage patients.
Owkin partners with global leaders such as Sanofi, Servier, Roche, and BMS to accelerate drug development through AI-based biomarker discovery, patient stratification, trial recruitment prediction, and real-world evidence modeling.
Using federated datasets across international research hospitals, Owkin helps pharma teams identify high-response subgroups, de-risk trial design, and uncover biological features linked to treatment outcomes.
3. LifeMine Therapeutics
LifeMine Therapeutics focuses on reinventing natural-product drug discovery by targeting diseases where nature’s chemical diversity offers a path to breakthrough therapies, particularly in oncology, inflammation, and infectious disease. Traditional natural-product discovery has remained slow, manual, and narrow in scope, leaving most of nature’s chemical space untouched.
LifeMine solves this through its Genomic Drug Discovery Platform, Avatar-Rx, that scans fungal DNA to find natural small molecules that already act on human disease targets, using fungi as “avatars” for these targets.
It looks for special gene clusters containing embedded target genes (ETaGs) that both make a molecule and encode a resistant version of the target, so researchers can predict what the molecule does without knowing its chemistry upfront.
This enables rapid, parallel target searches, delivers preclinical-ready candidates in about six months, and improves predictability and success rates compared with traditional small‑molecule discovery.

In 2022, LifeMine collaborated with GSK, granting the pharma giant access to its fungal genomics engine to develop next-generation small-molecule therapeutics. This deal includes R&D funding, potential milestones, and royalties, a strong signal of industry confidence in LifeMine’s approach.
4. ElevateBio
ElevateBio addresses one of the biggest bottlenecks in modern medicine: the difficulty of developing, scaling, and manufacturing cell and gene therapies.
To solve this, the company created ElevateBio BaseCampz.
BaseCamp provides a unified model for next-generation biomanufacturing, enabling teams to design, optimize, and scale therapies with seamless transitions from research to clinical production. Its capabilities span viral-vector production (AAV, lentivirus, retrovirus), cell therapy process optimization, QC/analytical testing, vector engineering, gene editing, and regulatory support.
By integrating these capabilities, ElevateBio reduces the high failure rates, long tech-transfer timelines, and fragmented workflows that traditionally slow advanced therapy development.
Beyond being a manufacturing powerhouse, ElevateBio also supports a growing internal pipeline across rare genetic diseases, immune conditions, and oncology. Simultaneously, it serves as a major development and manufacturing partner for biotech companies and academic institutions.
Further, ElevateBio’s “Life Edit” technology platform provides a comprehensive, flexible gene-editing toolbox to accelerate the development of cell and gene therapies. The platform emphasizes matching the optimal editing modality to each therapeutic challenge, leveraging a diverse collection of CRISPR systems and expertise.
The core technologies include Nuclease Editing, which cuts both DNA strands for gene insertion or deletion; Base Editing, which converts one nucleotide to another without a double-strand cut, enabling precise correction of disease-causing mutations; and Reverse Transcriptase (RT) Editing, also known as prime editing, which creates a single-strand break to replace an existing DNA sequence with a new one.
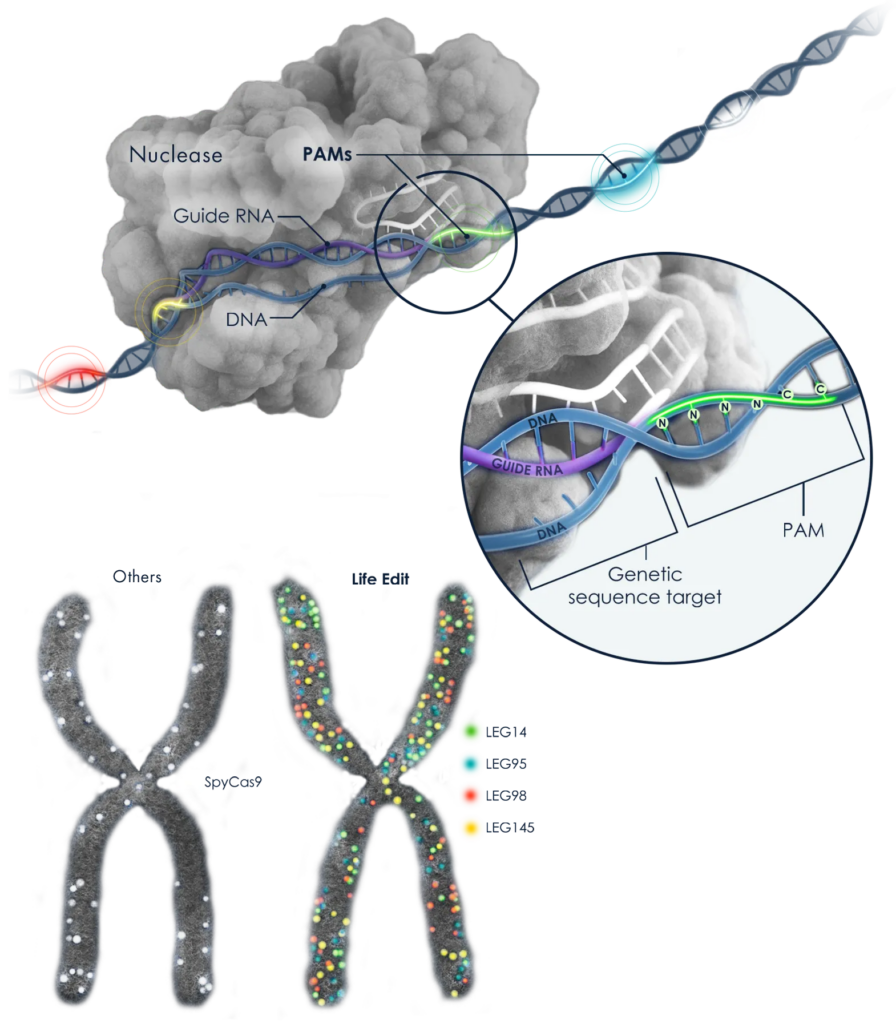
The platform also features a large panel of compact RNA-guided nucleases with diverse DNA recognition sequences (PAMs), providing broader genomic access and enhanced therapeutic delivery capabilities. This integrated approach, combined with AI and protein engineering, aims to unlock new possibilities for addressing a wide range of genetic disorders.
ElevateBio has been doing partnerships to improve its platforms and solutions. In 2025, it entered into a multi-year collaboration with AWS to significantly enhance its gene editing therapies.
By combining ElevateBio Life Edit’s extensive library of CRISPR systems and proprietary datasets with AWS’s powerful generative AI and cloud computing infrastructure, the company is accelerating the discovery and optimization of novel CRISPR drugs.
This alliance enables ElevateBio to train and deploy state-of-the-art protein language models at scale, enabling rapid design of custom CRISPR proteins.
5. Senti Biosciences
Once engineered cells enter the body, they are difficult to control. Senti Biosciences is currently tackling this major issue through its Synthetic Gene Circuit Platform, which embeds programmable biological logic into therapeutic cells.
Senti Bio’s Gene Circuit Technology Platform uses “intelligent genetic programming” to tackle complex diseases. This approach is like building tiny, smart computers inside cells to control how the therapies work.
These circuits offer four main capabilities:
- Multi-Arming, where one drug targets multiple disease pathways;
- Logic Gating, which helps the therapy accurately find and kill only the diseased cells;
- a Regulator Dial for turning the therapy up or down using existing oral drugs;
- and a Smart Sensor that precisely detects and reacts to the disease environment.
The platform aims to create next-generation treatments that are more precise and controllable.
Further, Senti Bio is developing Off-the-Shelf Natural Killer (NK) cell therapies for oncology, addressing limitations of traditional autologous approaches. NK cells are preferred due to their innate killing capability, potential for immune activation, and previously established safety profile in clinical settings.
The key advantage is the “off-the-shelf” nature, which allows the product to be manufactured in large, standardized batches from healthy donors, stored, and then rapidly delivered to a broad patient population in an outpatient setting, thereby improving patient access.
Senti’s strategy leverages peripheral blood NK cells, an existing supply chain, and mature GMP manufacturing processes, in partnership with a biofoundry, to efficiently produce clinical-grade, next-generation CAR-NK cell candidates.
Senti Bio’s pipeline is centered on Gene Circuit-Enhanced CAR Cell Therapy Programs for oncology. The wholly owned candidates include SENTI-202 for blood cancers such as AML/MDS, targeting CD33 and/or FLT3, and SENTI-301A for solid tumors such as HCC, targeting GPC3.
Additionally, the company is advancing collaboration programs utilizing its Gene Circuit technology in areas like Gene Therapy for eye, CNS, and liver diseases, and iPSC Cell Therapy for regenerative medicine.
6. Molecular Assemblies
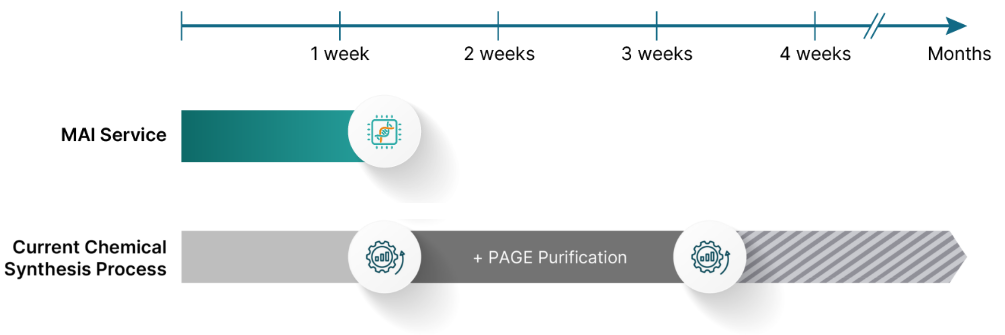
Molecular Assemblies, now part of TriLink Biotech, addresses the limitations of traditional chemical DNA synthesis, which produces short, less pure DNA sequences and struggles with complex structures like long homopolymer stretches. After nearly 50 years, this chemical method has plateaued in efficiency, resulting in costly, time-consuming purification steps.
To solve this challenge, the company is using Fully Enzymatic Synthesis (FES)™ technology. The Fully Enzymatic Synthesis (FES)™ technology uses enzymes to replace harsh chemicals in the synthesis of the DNA molecule.
Specifically, it uses a terminal deoxynucleotidyl transferase (TdT) enzyme to sequentially add one DNA base at a time to the growing oligonucleotide chain. This enzymatic process boasts a significantly higher stepwise incorporation efficiency (99.9%) compared to traditional chemical synthesis, resulting in purer final products.
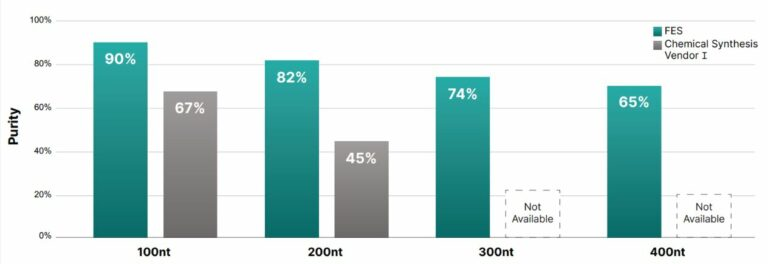
The synthesis occurs at neutral pH and at higher temperatures, which melt problematic secondary structures and facilitate the easy production of typically “difficult” sequences. Crucially, the process includes an in-process purification step, eliminating the need for lengthy, expensive post-synthesis purification and ultimately accelerating the delivery of long, pure DNA.
7. BenchSci
BenchSci addresses a critical bottleneck in drug discovery. The enormous volume of scientific literature, complex datasets, and scattered experimental records overwhelms researchers.
This consequently slows down the entire research process, introduces avoidable errors, and significantly reduces the reproducibility of foundational early-stage research.
The company solves this issue through ASCEND, an AI-powered platform that analyzes millions of peer-reviewed papers, reagent performance datasets, experimental outcomes, and biological annotations to identify which pathways, models, and reagents are truly validated for a specific target or disease.
Its primary function is to unravel complex disease biology by guiding scientists through the complexities of research, enhancing their decision-making, and boosting experimental productivity.
ASCEND achieves this by utilizing a robust knowledge base and multi-modal AI to process and harmonize massive amounts of data. This data includes scientific literature, patents, clinical data, internal pharma results, and more, effectively creating an evidence map of disease biology.
By decoding these insights, ASCEND helps scientists generate better research ideas and determine the most effective experimental paths to test them.
Pharmaceutical companies use ASCEND to support target selection, de-risk biological hypotheses, and improve experimental design at the earliest stages of R&D.
8. Orbit Discovery
Orbit Discovery specializes in peptide discovery solutions for therapeutic applications, including radiopharmaceutical targeting, nucleic acid delivery, and targeted protein degradation.
The core challenge the company addresses in drug development is the difficulty of efficiently performing high-throughput screening across the vast chemical space of potential peptide candidates. Traditional screening methods struggle to identify both peptides that bind strongly to a target (affinity) and, more importantly, those that exhibit the required biological function (e.g., activating or blocking a target).
Orbit solves this challenge with its proprietary Peptide Discovery Platform, which leverages a combination of a bead-based display system and microfluidics. In this system, each unique peptide is synthesized and displayed on the surface of a bead, with its DNA encoding sequence attached.
This setup allows the company to screen incredibly large libraries of diverse peptides rapidly.
The platform offers a significant advantage by enabling direct functional screening against reporter cells, which is crucial for identifying complex therapeutic leads, such as agonists and antagonists for cell-surface targets like GPCRs.
This high-diversity, high-throughput approach accelerates the identification of high-quality peptide hits that can be quickly moved into the therapeutic development pipeline.
Orbit Discovery further partners to expand research.
It collaborated with Evergreen Theragnostics to accelerate the development of targeted radiotherapeutics. The partnership leverages Orbit’s proprietary peptide screening platform to find and optimize novel targeting peptides, combined with Evergreen’s expertise in radiopharmaceutical development and clinical translation.
9. Neok Bio
Neok Bio is a biotechnology startup that is dedicated to transforming cancer treatment through the development of next-generation bispecific Antibody Drug Conjugates (ADCs).
It aims to overcome the significant limitations of conventional, first-generation ADCs, including a narrow therapeutic window, off-target toxicity, and limited efficacy against many solid tumors due to poor drug delivery and resistance.
Neok Bio solves this challenge by leveraging a dual-targeting strategy using its bispecific ADCs. Unlike traditional ADCs that target only one protein on a cancer cell, Neok’s platform engineers antibodies to simultaneously bind to two distinct, complementary cancer antigens (unique target pairs).
The startup’s advantage lies in its novel Topo-1-based platform, designed to develop superior bispecific cancer treatments. This platform intelligently combines carefully selected cancer targets and antibody structures with advanced payload and conjugation technology.
The key technology used is the proprietary SYNtecan E™ linker-payload technology, which ensures the drug remains stable until it reaches the tumor. The payload, Exatecan (a potent Topoisomerase-1 inhibitor), is chosen for its superior properties, including its ability to kill neighboring cancer cells (a greater bystander effect) and to reduce the risk of drug resistance. This comprehensive approach allows the company to fine-tune its bispecific format for each specific cancer target pair.
The startup is advancing a pipeline of these differentiated bispecific ADCs, including lead candidates NEOK001 and NEOK002, targeting proteins highly expressed in various solid tumors, aiming to achieve superior safety and efficacy profiles.
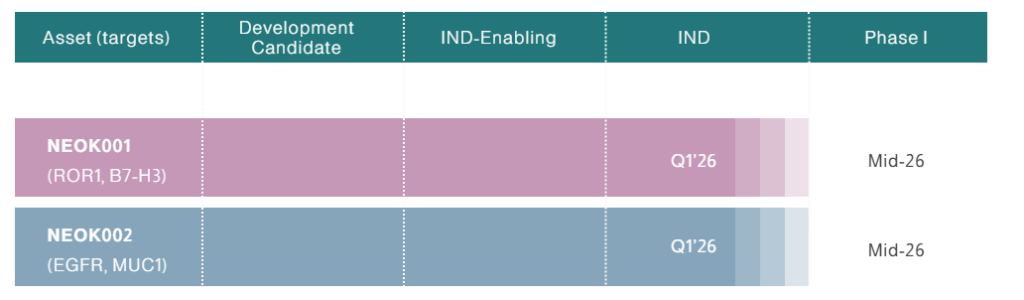
10. Peptilogics
Peptilogics focuses on reimagining the discovery of peptide therapeutics for patients with serious and life-threatening diseases. The challenge it tackles is the limited scale and precision of traditional drug design, which hasn’t fully explored the therapeutic potential of peptides.
Peptilogics solves this by combining the untapped power of peptide biology with machine learning in its proprietary AI platform, Nautilus. The platform utilizes proprietary Novel AI Algorithms—both generative and predictive—which learn from harmonized public and proprietary data to design and optimize peptides in silico (through simulation).
To execute this at speed, it employs a supercomputer for rapid data processing and model training. By integrating these computational tools with custom Peptide R&D synthesis and rich data collection, the platform enables multiparameter optimization, efficiently engineering desired drug-like properties into new peptides, and accelerating time-to-discovery.
This approach efficiently explores new chemical space to engineer peptides with optimized properties (e.g., binding, selectivity, and safety), thereby accelerating the development of next-generation medicines.
Peptilogics’ pipeline focuses on developing peptide therapeutics for areas with high unmet patient needs, with its lead candidate, PLG0206.
PLG0206 is an investigational antibiotic peptide designed to treat Periprosthetic Joint Infection (PJI), a severe, life-threatening condition where standard antibiotics often fail due to bacterial biofilms.
PLG0206 is a fast-acting, broad-spectrum peptide that utilizes a unique mechanism to disrupt bacterial cell membranes and has anti-biofilm activity. Following acceptable Phase 1 safety data and FDA designations (Orphan Drug and QIDP) for PJI, it is currently in clinical trials.
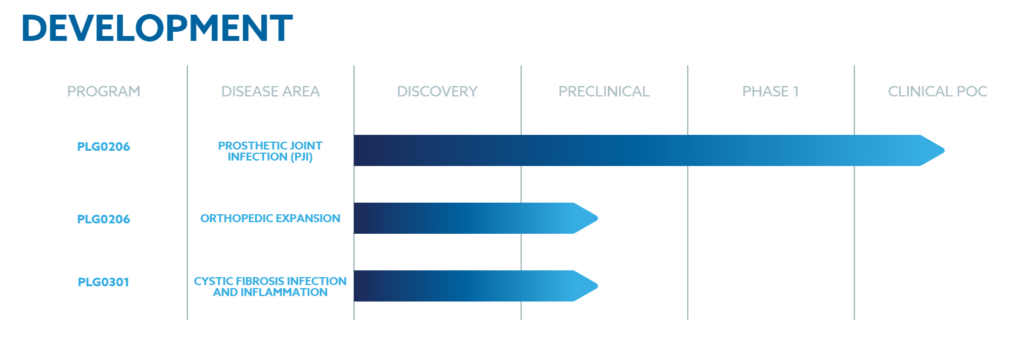
Beyond PJI, the startup is advancing other programs, including PLG0301 for Cystic Fibrosis infection and inflammation, as well as multiple early-stage oncology and rare genetic disease programs, all leveraging its powerful AI discovery platform.
Conclusion
The startups profiled here collectively signal a fundamental shift in pharmaceutical R&D—from empirical, resource-intensive processes to precision-engineered, data-driven platforms.
As AI-discovered drugs like Insilico’s Rentosertib advance through mid-stage trials and gene-editing collaborations accelerate through cloud computing partnerships, these companies are establishing new standards for speed, cost-efficiency, and therapeutic precision.
Their influence extends beyond individual pipelines: by demonstrating that complex diseases can be addressed through federated learning, enzymatic synthesis, programmable cell circuits, and fungal genomics, they’re validating entirely new categories of druggable targets and modalities.
Major pharma partnerships with companies like Sanofi, GSK, Roche, and BMS underscore industry recognition that platform-driven innovation now rivals traditional discovery models.
As these technologies mature and reach commercial scale, the cumulative impact will likely reshape pharma’s competitive landscape, making precision medicine accessible across oncology, rare diseases, and chronic conditions that have historically resisted treatment.
How Can We Help You?
We support industry-leading R&D and Innovation professionals through complex problems. Describe your challenge, and let us bring clarity and expertise.