Value Delivered
The findings presented a strong argument against the patent’s claims. They demonstrated that similar enzymes in prior art not only had the necessary variations but also showed increased stability and activity.
This evidence formed a compelling case during the patent opposition process, ultimately leading to the invalidation of the key claims.
Problems Solved
The task was to identify specific variations and substitutions within enzyme sequences that were around 500 amino acids in length. These variations were supposed to lead to increased stability in the presence of preservatives. Initially, there were references suggesting that modifying enzyme sequences generally resulted in decreased activity, which contradicted the patent’s claims of enhanced stability and activity.
Additionally, it was also challenging to pinpoint slight amino acid variations that had a technical effect, as this required extensive examination of biochemical literature and databases that often lacked direct comparative data between human and non-human enzyme sequences.
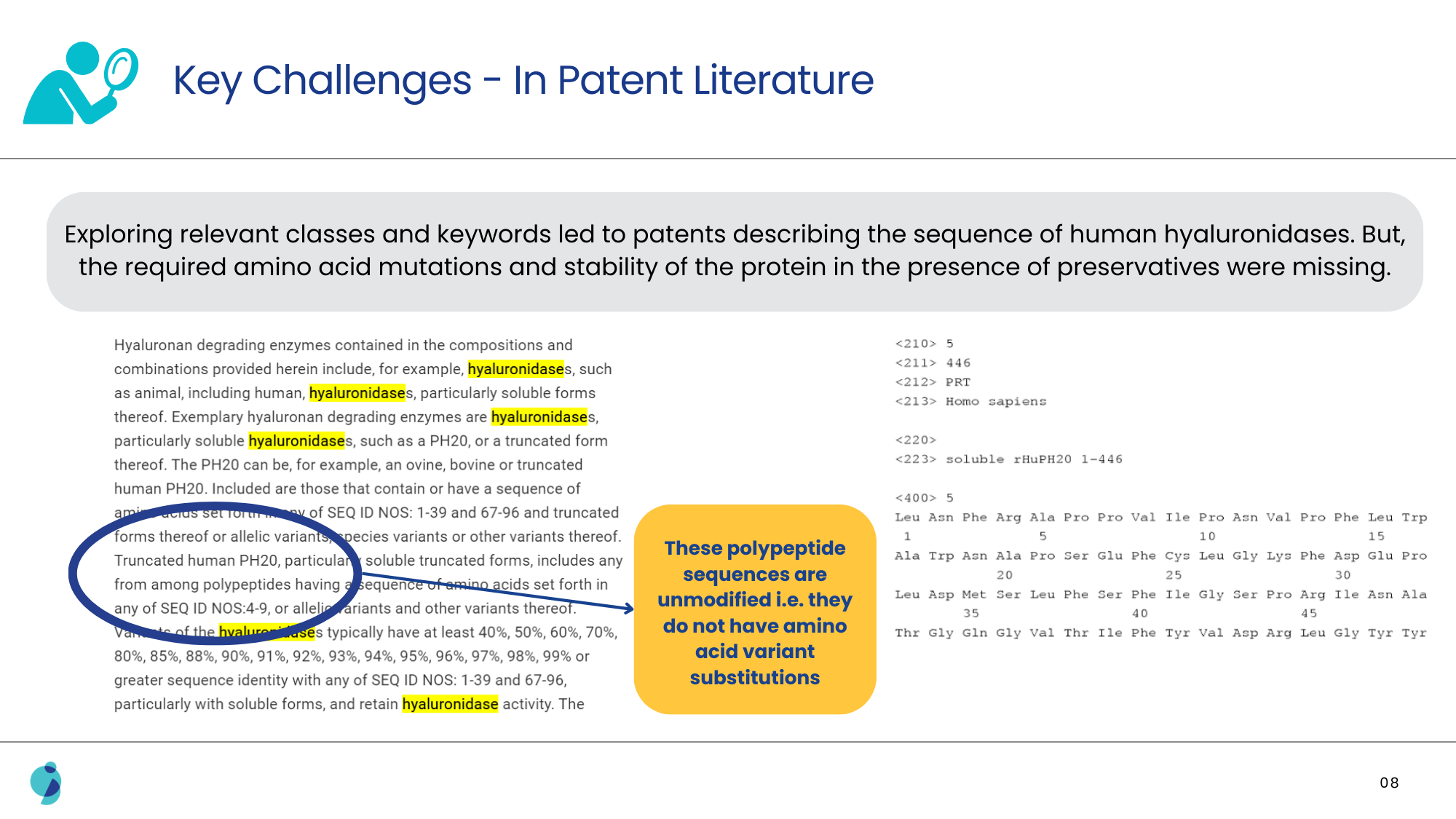
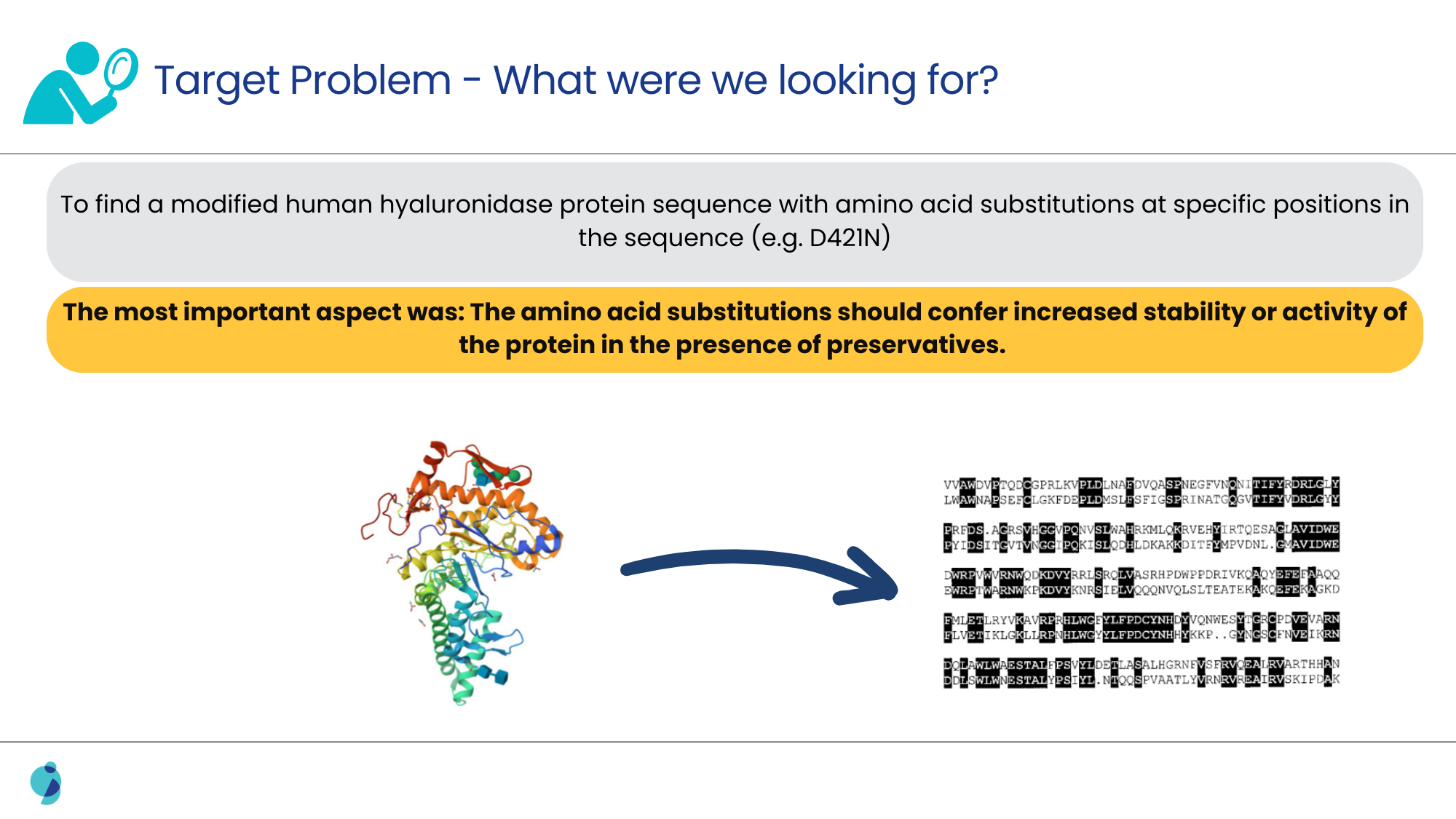
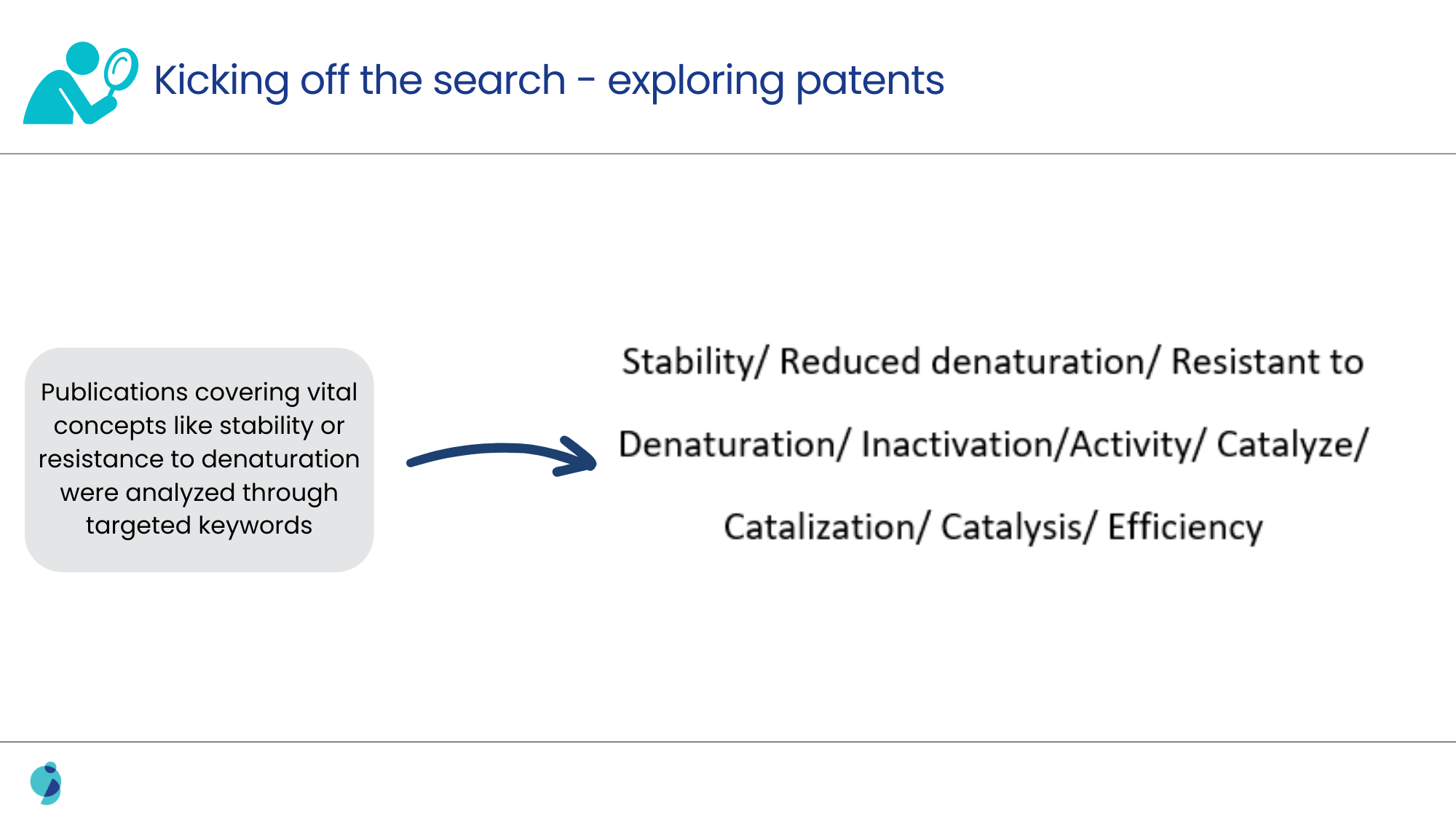
Solution Offered
A targeted sequence search identified several non-human enzyme sequences having the required amino-acid variations. To assess the potential technical effect of these modifications, literature comparisons between non-human and human enzymes were explored. This exploration yielded crucial insights, revealing that non-human enzymes possessed the desired sequence variations and demonstrated greater stability and activity than their human counterparts.
Evidence from these comparative studies was instrumental in challenging the novelty of the patented claim by highlighting that the supposed technical improvement was already evident in existing knowledge.
Get the full case study to discover how GreyB successfully invalidated a biotechnology patent related to modified enzyme sequence.
Request Full Case Study
Download in PDF Format and read anytime. Fill the form to get access to this article.