Stay ahead in generic drug launches with
time-sensitive notifications
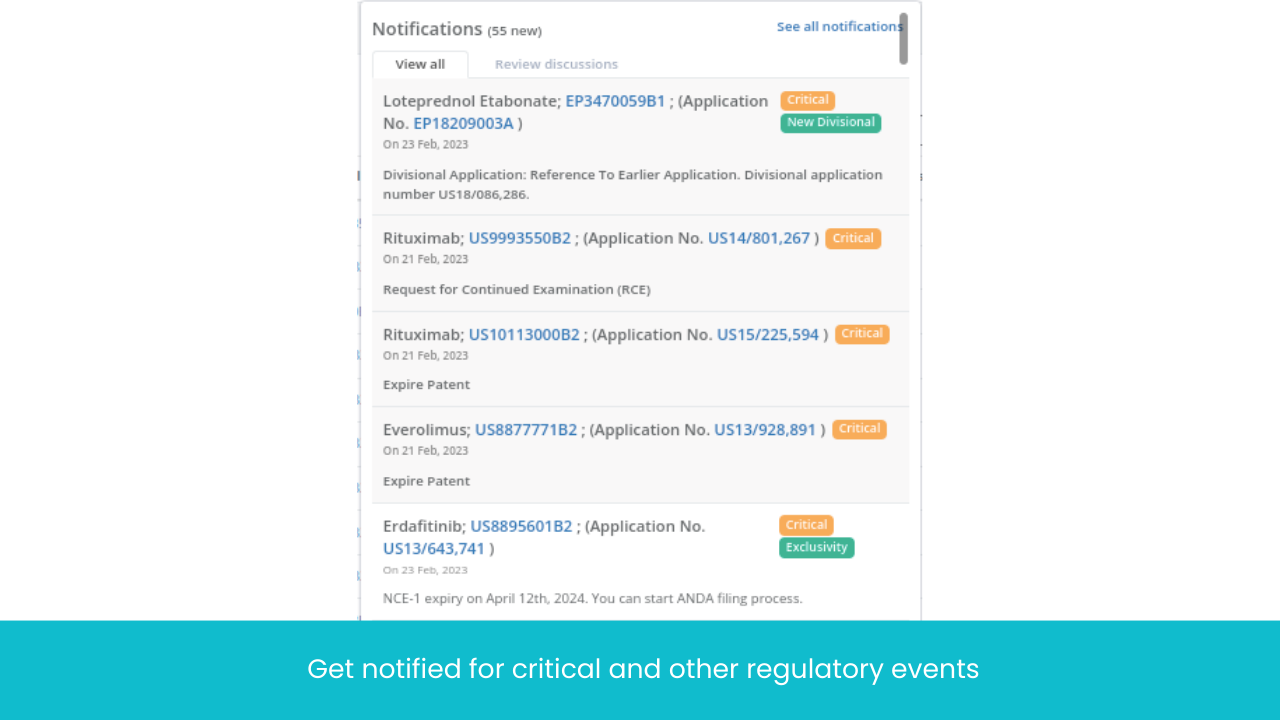
Get instant alerts for patent lifecycle events that impact the generic launch like Notice of allowance, Abandoned, Oppositions, NCE1 from different regulatory like USPTO, EPO and Orange Book under one place
Over 1000+ Users and 100+ Brands Trust GreyB






























Why you need Elixir?
Missing out important events that impact generic launch
Inbox choked with alerts from different jurisdictions
Common language for all the events
Countless excel files shared across email for collaboration
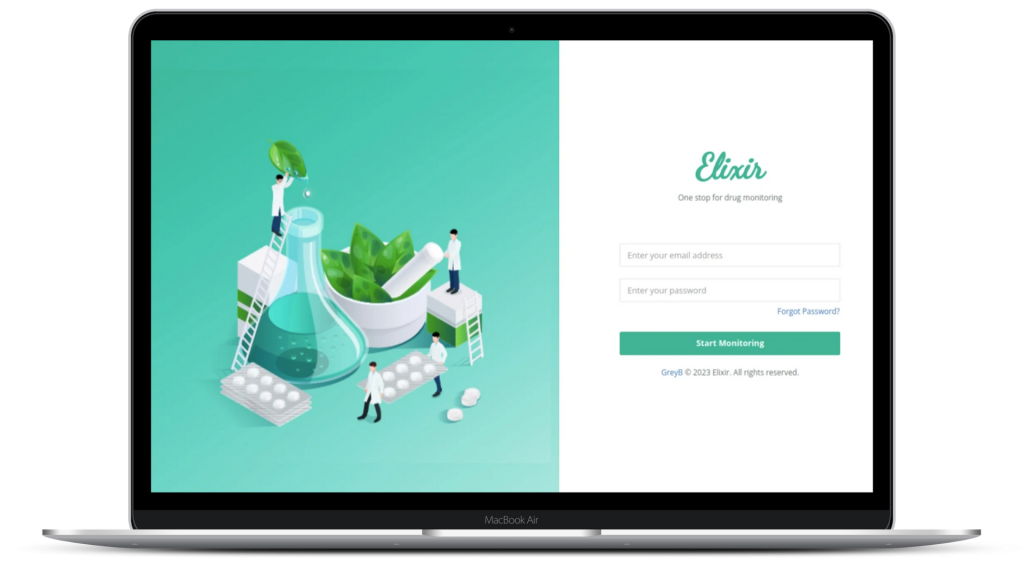
What does Elixir track?

Track important events of patent lifecycle globally in one place
Context aware alerts so you never miss crucial regulatory deadlines (like oppositions, ANDA filings, and Paragraph IV Litigation)
Intelligent categorization of events for effective drugs prioritization (Elixir bring your attention to important information only)
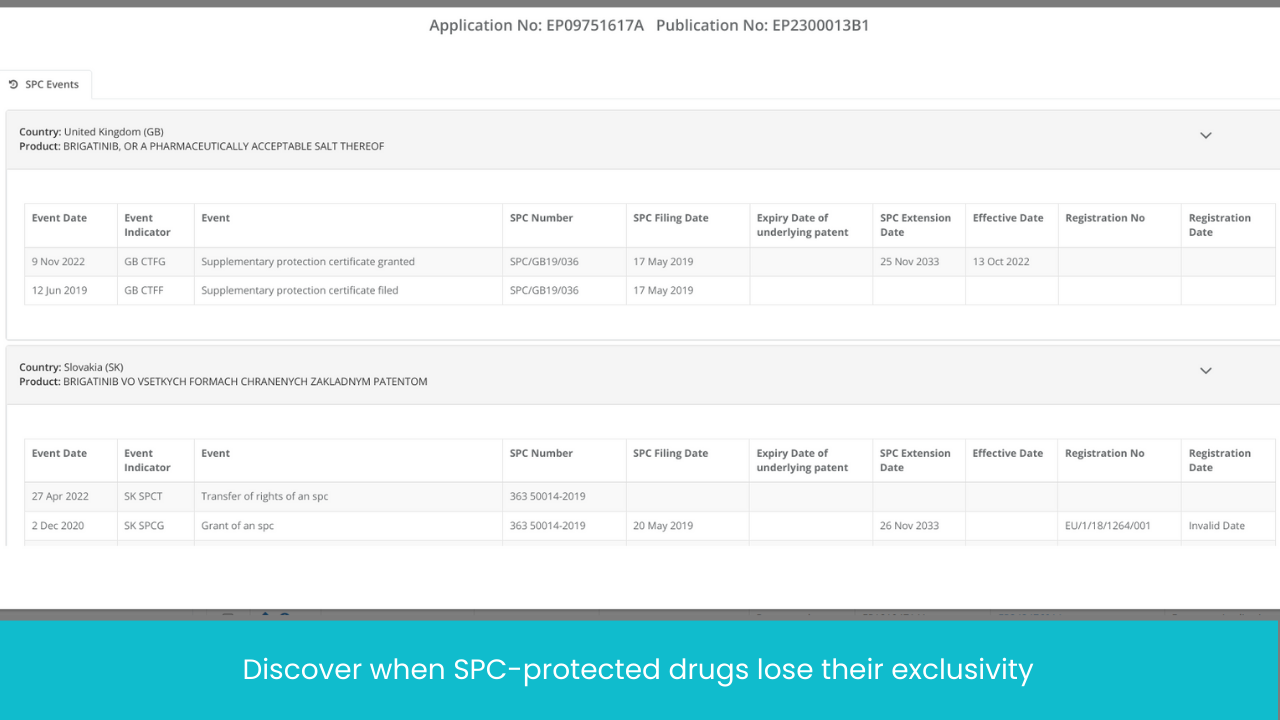
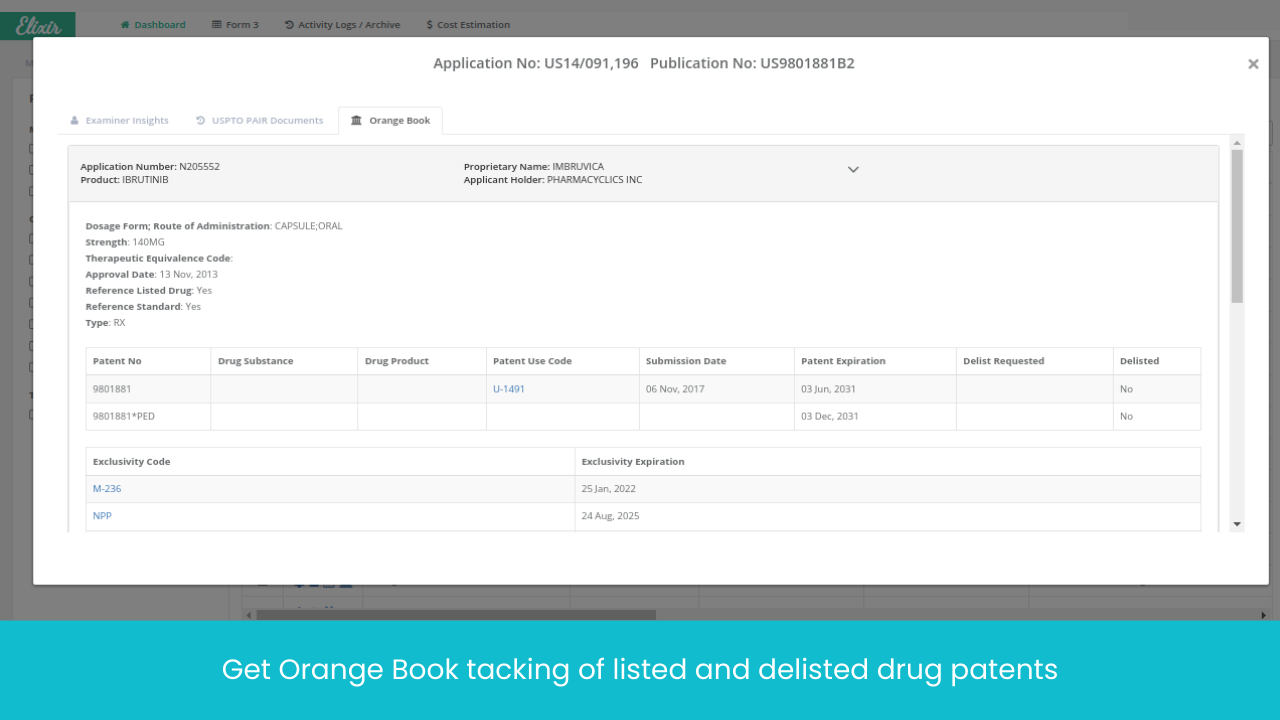
No more database hopping! EP, US and 20+ jurisdictions patent tracking, SPC tracking, all under one place
All events translated to one common language (Notice of Allowance = Intention to grant)
How Elixir solves the existing problems with a centralized system and an email
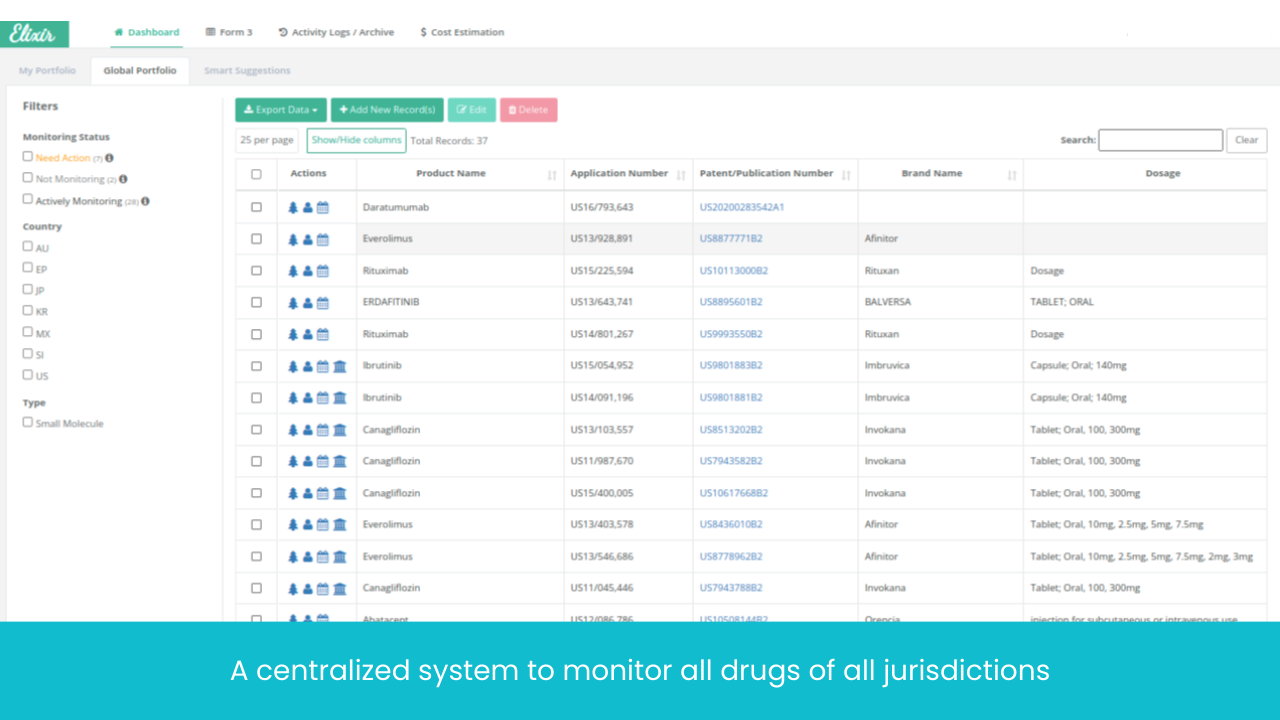
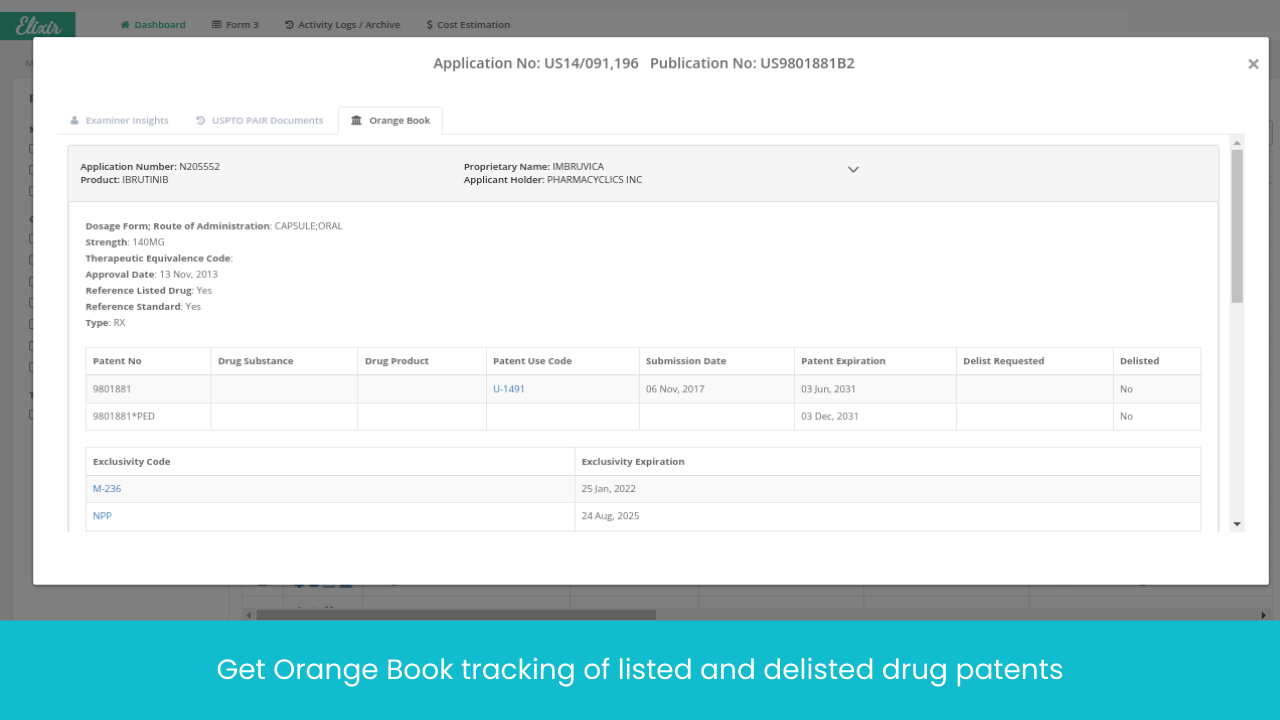
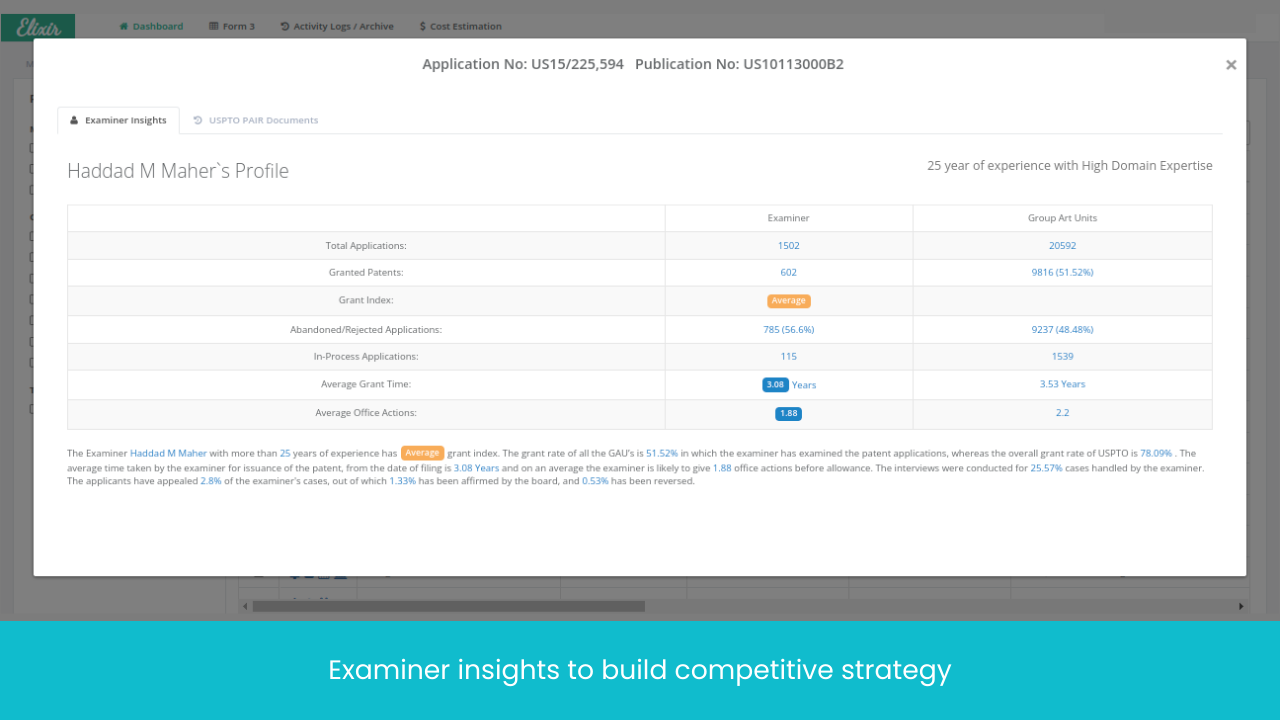
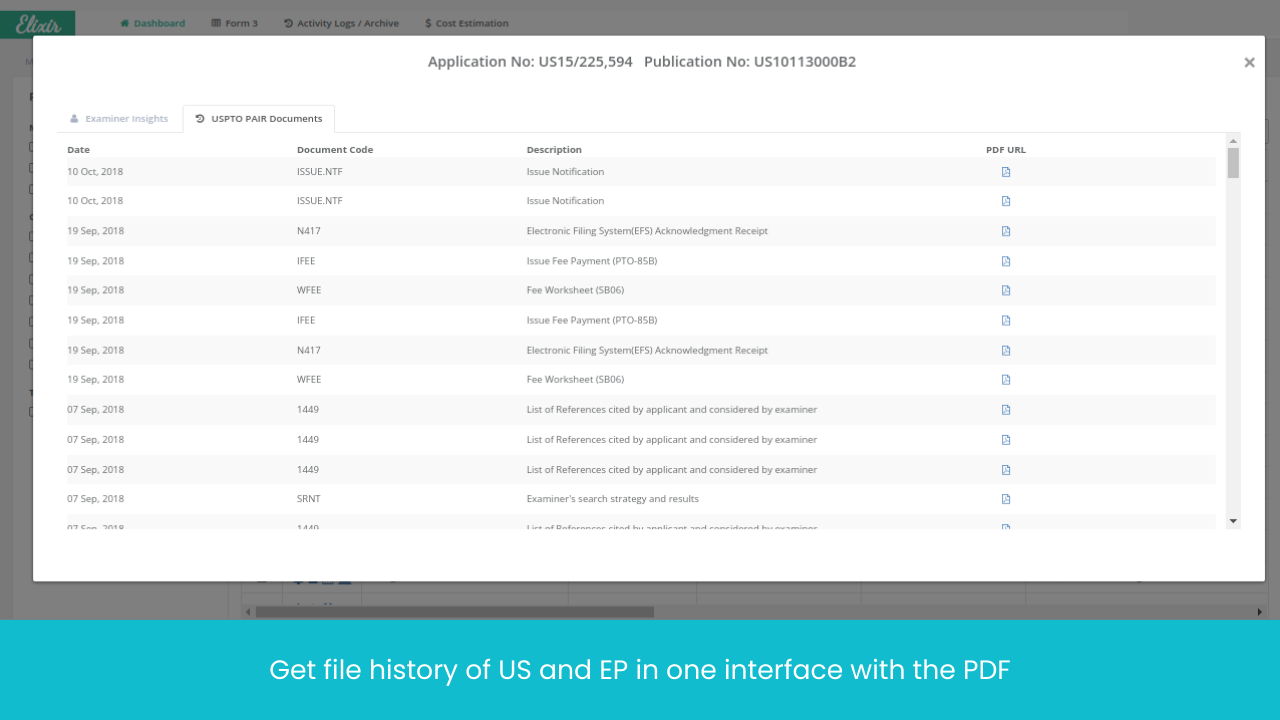
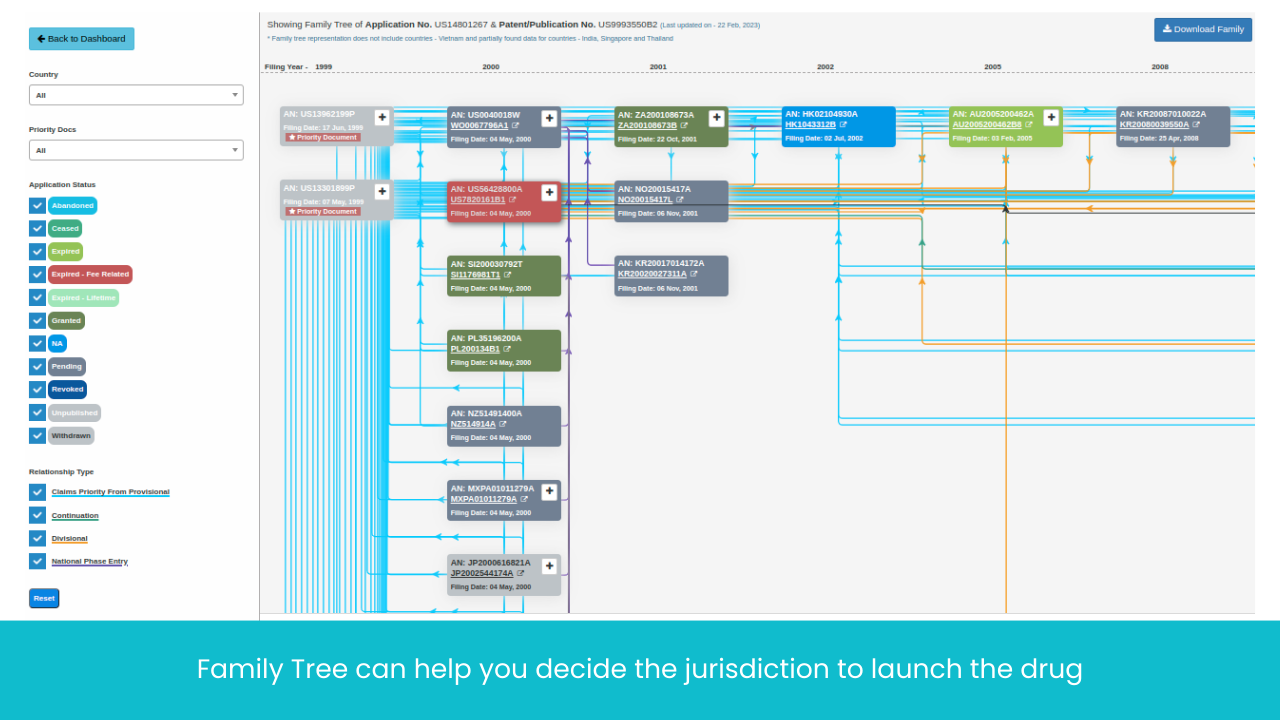
Monitor Patents Divisionals /Continuations/ Continuations-in-Parts protecting the Target drug
Tired of using Excel for your tracking activities
Proceedings before the Unified Patent Court (UPC)
Paragraph IV Litigation
Identifies 180 days of market exclusivity opportunities
Monitors competitors through citations and their market activities
Sign up for your free trial today
Elixir vs Other Drug Monitoring
|
|
GreyB Elixir
|
Other Tools
|
|---|---|---|
|
Highly Customizable
|
|
|
|
Optimized Notifications of Critical Events
|
|
|
|
Automation + Manual Intervention
|
|
|
Elixir monitors US, Europe, and 50+ countries
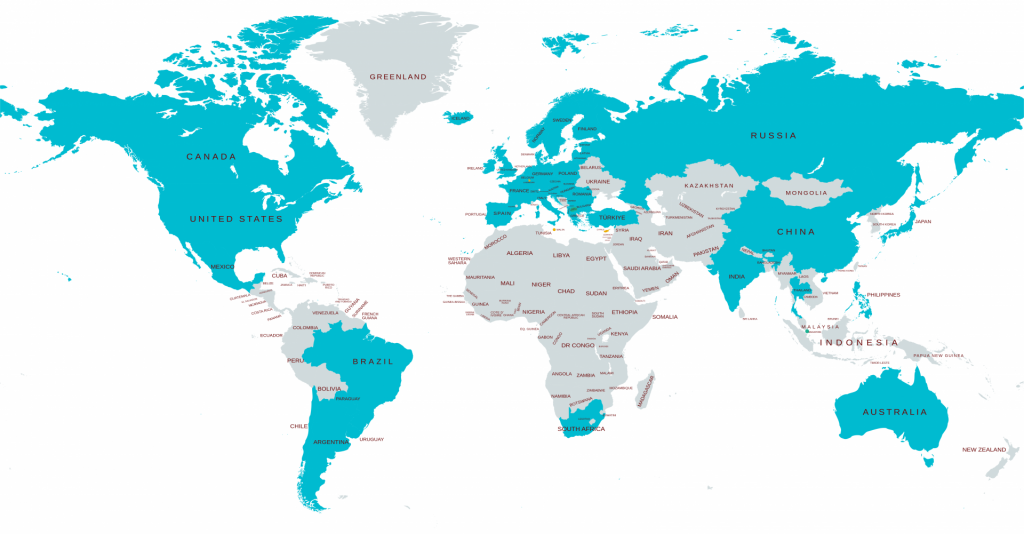
Build your Drug Launch Strategy with Elixir
How Elixir helped companies like yours?
“Optimised notifications are useful to see all jurisdictions events”
|
“Elixir team provides a fast solution for any customisation”
|
“Critical events notification on same day with the next action to take”
|
“Best tool for monitoring drug patents in a centralized system”
|
Case Studies Related To Tools
Most Asked Questions
How does a drug monitoring tool like Elixir work?
What types of information does Elixir track?
Who can benefit from using a drug monitoring tool like Elixir?
How does Elixir solve the problems associated with using spreadsheets?
Can Elixir be customized to fit specific business needs
How can Elixir help with making informed management decisions?
Is Elixir suitable for both bio-pharmaceutical and small-molecule drugs?
How does Elixir contribute to staying ahead of the competition?
How long do drug patents last?
Usually 20 years.
A drug patent expires 20 years from the date on which the patent application was filed in the United States. Sometimes, a term extension is provided if the original patent was delayed due to secrecy orders, interferences, or appellate review periods.
Generics can be launched once the patent-protected drug surpasses the patent expiry and exclusivity date.
Elixir provides the drug patents list expiring in a particular year, like 2023, 2024, and more.
Why is drug monitoring important?
What is your privacy policy on data? Is my data safe?
- We won’t use your data in any hidden ways for monetization or expansion activities. You own your data. We are ISO 27001 certified and SOC-2 compliant. Refer to our privacy policy.
- To ensure the safety of your data, we keep 3 months of backup in a geographical location to ensure no disaster can impact your data and operation.
Do I need to train my staff?
What are the geographical location of your servers?
What happens in case of any disaster?
How do I reach out to you in case of some query?
The support team is reachable at [email protected]; our response time is within 24 hours. You also get a dedicated support team with the package for faster response time.








