Neurological disorders, ranging from Alzheimer’s disease to Parkinson’s and multiple sclerosis, present significant challenges in the medical field due to their complex nature and the brain’s intricate structure. One of the main obstacles in treating these disorders is the blood-brain barrier (BBB), a selective semipermeable border that protects the brain from foreign substances.
While this barrier is crucial for maintaining the brain’s internal environment, it also restricts the passage of most therapeutic agents, including large and small-molecule drugs. These molecules often fail to reach therapeutic concentrations in the brain, rendering them ineffective in treating central nervous system (CNS) disorders.
Furthermore, the specificity of these drugs is another concern; they must target the affected brain regions or pathways without affecting the rest of the CNS, reducing the risk of side effects.
Why are the Current Drugs Not Able to Cross the Blood-Brain Barrier?
Current therapeutics, especially larger and more complex drug molecules, often struggle to pass through the blood-brain barrier (BBB) for several reasons, even when using advanced delivery techniques:
Size and Structure: Many traditional drugs are larger and have more complex structures than RNA molecules. This makes it difficult for them to pass through the tight junctions of the BBB, even when using nanoparticle carriers or other delivery methods. The BBB is very selective, allowing only certain sizes and types of particles to pass through.
Biochemical Properties: Drugs often have different biochemical properties, such as solubility and charge, affecting their ability to cross the BBB. RNA therapeutics can be designed specifically to have properties that facilitate crossing the barrier, whereas traditional drugs might not have these adaptable properties.
Targeting and Specificity: RNA therapeutics can be engineered to target specific cells or receptors in the brain, enhancing their ability to be transported across the BBB. Traditional drugs may lack this high level of specificity, making it harder for them to be recognized and transported by the brain’s natural transport systems.
Immune Response: Some therapeutic agents can trigger the immune system’s defense mechanisms, leading to inflammation or other reactions that can further tighten the BBB. RNA therapeutics can often be designed to avoid such immune responses, thereby having a better chance of crossing into the brain.
Degradation and Stability: Traditional drugs might be more prone to degradation in the bloodstream before they even reach the BBB. RNA therapeutics can be encapsulated or modified to protect them until they reach their target, enhancing their stability and efficacy.
RNA Therapeutics: A Promising Approach
RNA therapeutics, particularly those based on small interfering RNA (siRNA) and messenger RNA (mRNA), offer a novel and promising approach to circumvent the above challenges.
Unlike traditional drugs, RNA therapeutics can be designed to target the genetic underpinnings of neurological disorders specifically. They work at the molecular level to modulate gene expression by silencing harmful genes or promoting the expression of beneficial proteins. This specificity enhances the therapeutic potential and minimizes the risk of off-target effects.
How can RNA therapeutics able to cross BBB?
RNA therapeutics can cross the blood-brain barrier (BBB) through various mechanisms.
One approach to enable the crossing of RNA therapeutics across the BBB is to re-engineer the biologic with BBB drug delivery technology. This involves modifying the RNA molecule or using carrier systems that facilitate transport across the BBB. For example, antibody-based carriers have been developed to utilize the endogenous macromolecule transportation pathway known as receptor-mediated transcytosis, which can enhance BBB crossing.
Another strategy is to identify and target specific receptors on the BBB that are more specific to the brain. This can help minimize fast clearance from the blood and increase the concentration of therapeutics inside the brain. Ongoing research aims to identify novel receptor-mediated transcytosis (RMT) targets that can enhance BBB crossing efficiency.
What challenges hinder the widespread adoption of RNA Therapeutics in Neurological Disorders?
The widespread adoption of RNA therapeutics in treating neurological disorders faces several challenges:
Delivery and Targeting: Efficiently delivering RNA therapeutics to the brain remains a significant hurdle. Crossing the blood-brain barrier (BBB) is a complex task, and ensuring these therapies reach the specific brain region and cells affected by the disorder is challenging. Developing delivery systems that can reliably and safely transport RNA molecules into the brain without being degraded or causing unintended effects elsewhere in the body is crucial.
Stability and Durability: RNA molecules are inherently unstable and can be quickly degraded by enzymes in the body. Enhancing the stability of RNA therapeutics without compromising their safety and efficacy is essential. Furthermore, for long-term treatment of chronic neurological disorders, the therapeutic effects of RNA molecules need to be durable, requiring either stable and long-lasting formulations or repeated administrations.
Off-Target Effects and Safety: To minimize off-target effects, RNA therapeutics must be highly specific to the target genes or proteins associated with a neurological disorder. Unintended interactions with other cellular components can lead to side effects and reduce the treatment’s overall safety. Thorough understanding and testing of the RNA molecule’s interaction within the complex environment of the brain are necessary to ensure safety.
Cost and Accessibility: The development and production of RNA therapeutics can be expensive due to the need for specialized technologies and manufacturing processes. These costs can limit patient accessibility, especially in lower-income regions or healthcare systems with limited funding for advanced therapies.
Regulatory Hurdles: RNA therapeutics represent a relatively new class of treatment, and regulatory agencies may require extensive data to demonstrate their safety, efficacy, and long-term effects. Navigating the regulatory landscape can be time-consuming and costly, potentially delaying the availability of these treatments to patients.
Public Perception and Education: As a novel treatment modality, RNA therapeutics may face skepticism from the public and healthcare professionals. Educating stakeholders about the benefits and limitations of RNA-based treatments is essential for their acceptance and appropriate use.
Research and Development Challenges: Understanding the complex pathophysiology of neurological disorders at the genetic and molecular level is critical for designing effective RNA therapeutics. This requires extensive research and collaboration across disciplines, which can be resource-intensive and require significant investment.
However, there has been some progress as some startups find innovative approaches to deal with these challenges.
Startups Pioneering RNA Therapeutics for Neurological Disorders
Several startups are at the forefront of developing RNA therapeutics for neurological disorders, showcasing the potential of this technology:
1. NEUmiRNA
NEUmiRNA is a pioneering startup harnessing the power of antisense oligonucleotides to develop innovative treatments for neurological conditions such as epilepsy. Their cutting-edge technology involves delivering precisely designed antisense oligonucleotides to the brain, where they can effectively inhibit problematic enzymes like Adenosine Kinase (ADK).
By blocking these enzymes, NEUmiRNA’s approach aims to correct the underlying molecular imbalances contributing to neurological diseases. This novel drug delivery method overcomes one of the major challenges in treating brain disorders – the inability of many conventional drugs to cross the blood-brain barrier and reach their intended targets.
NEUmiRNA’s antisense oligonucleotides are engineered to penetrate this protective barrier, ensuring that the therapeutic agents can act directly on the affected brain regions. With this technology, the startup is paving the way for more effective and targeted treatments, thus offering new hope to patients suffering from debilitating neurological conditions like epilepsy.
In Oct 2023, NEUmiRNA collaborated with Motac Neuroscience to develop groundbreaking RNA therapies poised to revolutionize Parkinson’s Disease treatment.
Motac offers specialized preclinical services to pharma companies, supporting the discovery and development of therapeutics targeting neurological and psychiatric disorders.
This collaboration is another proof that NEUmiRNA’s solution is promising.
2. Crucible Therapeutics
Motor Neuron Disease (MND) and Frontotemporal Dementia (FTD) are neurodegenerative disorders that currently lack curative treatments. MND progressively leads to the degeneration of motor neurons, resulting in paralysis and mortality within a few years of onset.
FTD, conversely, selectively targets the frontal and temporal lobes of the brain, manifesting as personality changes, language impairments, and movement disturbances. Both conditions represent significant unmet medical needs, as there is a critical dearth of effective therapeutic options.
Crucible Therapeutics, a spin-out company from the University of Sheffield, has emerged as a promising player in the quest to develop treatments for MND and FTD cases caused by the C9orf72 genetic mutation.
This particular mutation disrupts the production of functional RNA molecules and generates harmful RNA species that contribute to neuronal degeneration and cellular damage within the brain.
Crucible Therapeutics’ innovative approach addresses this root cause by targeting the SRSF1 protein. This protein can bind to and remove deleterious RNA molecules from the cell nucleus, potentially mitigating cellular damage. This strategy holds the potential to offer a novel treatment modality for MND and FTD by directly addressing the underlying genetic etiology rather than merely managing the symptoms.
In September 2023, the startup raised £5M from Northern Gritstone and Argobio Studio to fund essential development, leading to its first clinical trial.
3. Vico Therapeutics
VICO Therapeutics tackles neurological disorders with a new approach: RNA modulation.
Our genetic code is transcribed into RNA, a blueprint for protein production. In neurological diseases, mutations can disrupt this process, creating abnormal proteins that damage nerve cells.
VICO Therapeutics’ weapon of choice is antisense oligonucleotides (AOs), tiny synthetic RNA molecules designed to target specific RNA sequences.
Their VICOMER™ platform offers an arsenal of ways to manipulate RNA. AOs can block the translation of harmful proteins, essentially preventing their creation. They can also correct RNA splicing, ensuring proper protein assembly or even editing mutations within the RNA.
Additionally, the platform allows for RNA degradation, eliminating harmful molecules or even activation, boosting the production of beneficial proteins.
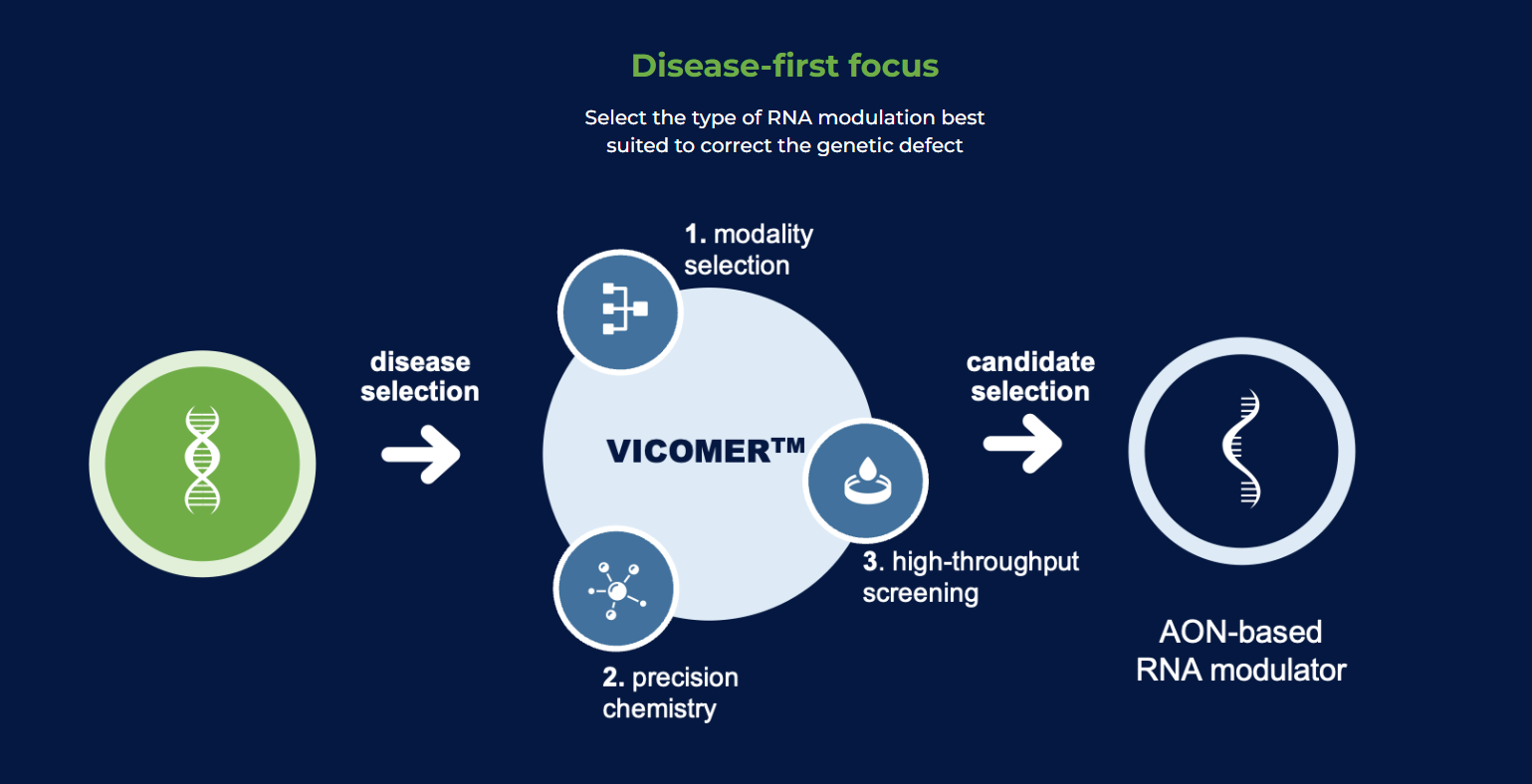
This versatility allows VICO Therapeutics to tailor the RNA modulation approach to each patient’s genetic defect. By targeting the root cause of the disease at the RNA level, this personalized medicine approach can potentially be more effective and safer than traditional therapies.
The startup has raised $90 Million in funding. Their latest round of funding was Series B, in which they raised €54M from Ackermans & van Haaren.
Ackermans & van Haaren is a diversified company operating in 5 sectors. Out of 11 investments, It was a lead investor in only three companies, including VICO Therapeutics.
Future Outlook
The future of RNA therapeutics is promising, especially in addressing complex neurological disorders. Startups and research institutions are at the forefront, driving innovations that could revolutionize treatment methodologies.
Developing RNA therapeutics has historically been challenging for pharma companies due to high costs and technical complexities. However, the emergence of startups specializing in RNA therapy development is paving the way for more efficient and cost-effective solutions.
Nevertheless, the current landscape is fragmented, with different startups and research groups focusing on distinct aspects of the RNA therapeutic development pipeline. Identifying and integrating the most promising solutions from these diverse sources can be daunting for those seeking to develop RNA therapeutics for neurological diseases.
This is where scouting companies like GreyB play a crucial role, offering expertise in pinpointing the startups and academic institutions making headway in targeted areas of RNA therapy.
This collaboration is essential for overcoming the existing barriers and advancing the development of RNA-based treatments for neurological diseases.
Learn more about startup scouting services Or reach out to our Innovation Experts:
Author: Simran Gohan and Vikas Jha, Solutions.