Current drug delivery methods usually have a short duration of action. This means patients need to take frequent dosages. Many individuals with chronic illnesses find this inconvenient, making it difficult to adhere to their treatment plans. Research indicates that adherence rates can be as low as 50%, as the frequent dosing required can be quite burdensome.
There’s also a risk of systemic toxicity or reactions because conventional drug delivery systems often affect unintended areas of the body.
But what if there were delivery systems that carry specially encapsulated drugs to target sites and release the medicines periodically? Some inventors have achieved precisely this result using nanotechnology and ‘soft’ robots. These carrier systems are administered to patients only once. They release the drugs over a prescribed period, making therapies more convenient and effective.
This report describes how leading companies are investing in nanotech-based robotic compounds for the drug delivery systems market. To get the complete report on emerging drug delivery solutions, please fill out the simple form below.

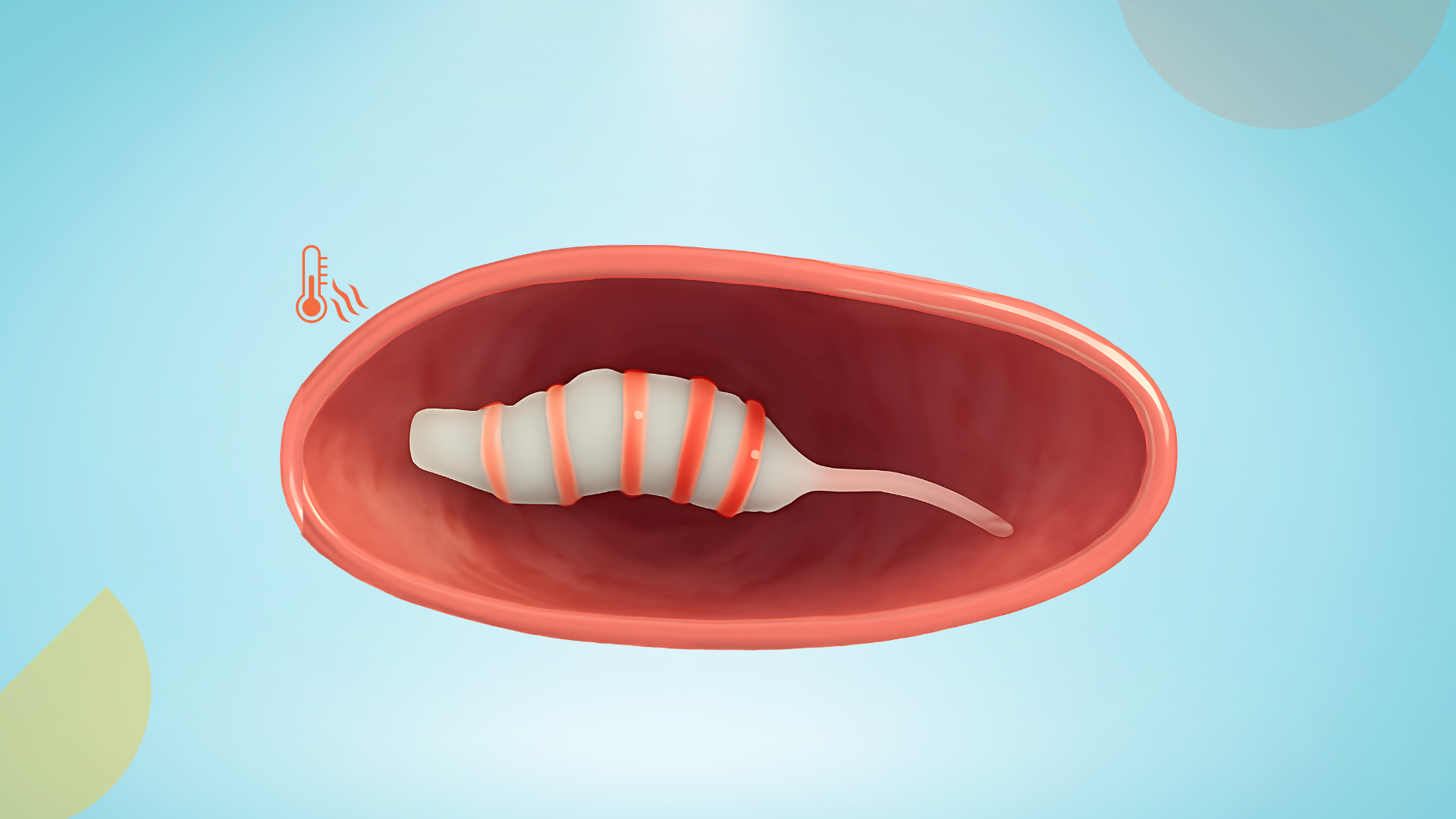
Johns Hopkins made a gelatinous drug-robot powered by body temperature.

Researchers at Johns Hopkins have developed a ‘gelbot’ inspired by the design of an inchworm. The 3D-printed drug carrier responds solely to changes in temperature, pH, or light to move to the target area in the body. In addition to delivering drugs, it can also carry imaging sensors to the required location with minimally invasive procedures.
Their robot’s patented design consists of multiple segments, each made of two layers. This includes an active layer that swells and shrinks in response to environmental stimuli (such as temperature), and a passive layer that remains stable, allowing the active material to interact with the surface it moves across.
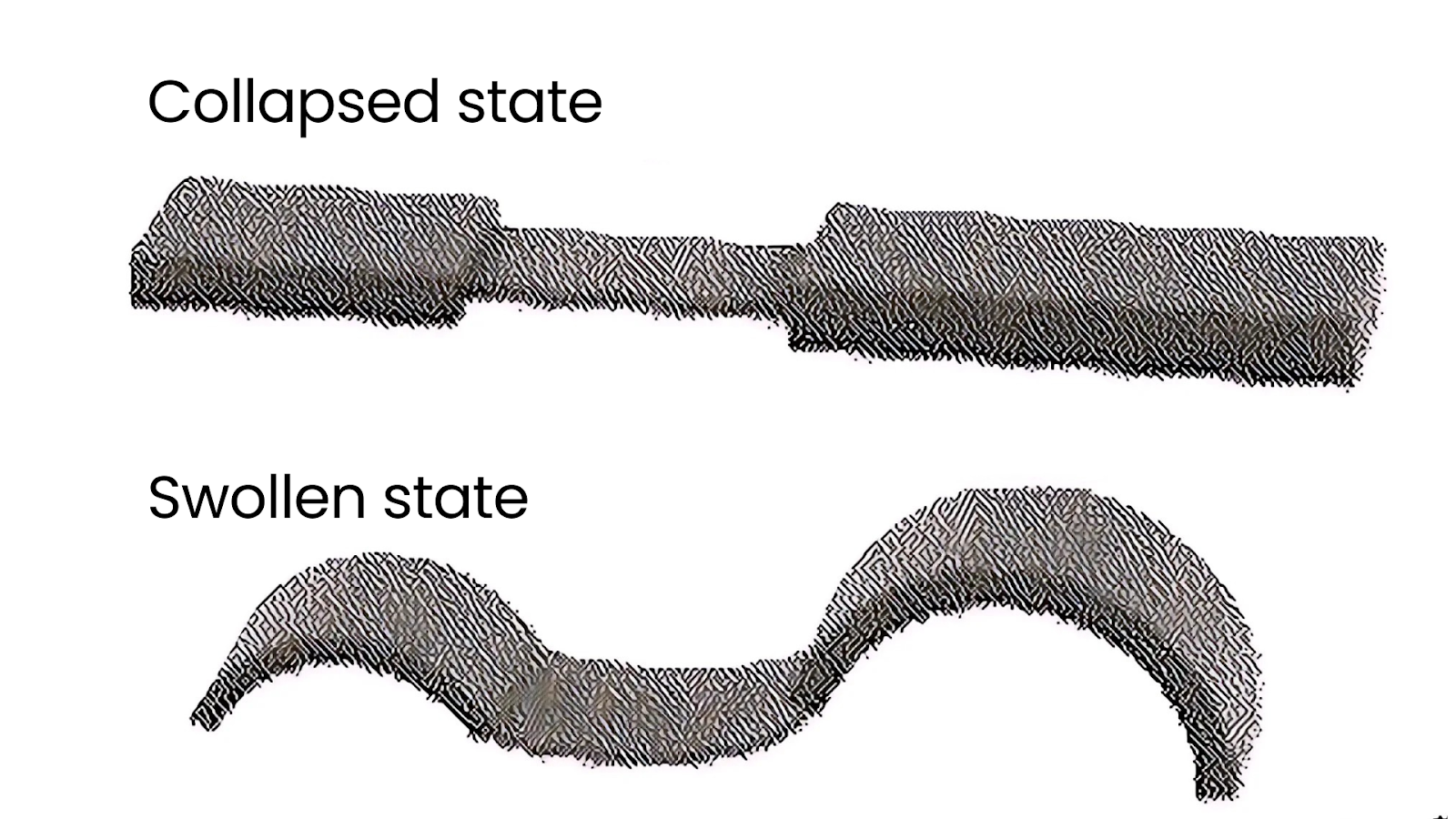
The robot moves by changing its shape. When it cools down, the materials inside swell, making it bend. Conversely, when it heats up, the materials shrink, allowing it to straighten out. One part of the robot creates more resistance, which helps it crawl forward. By varying the stiffness and asymmetry of the robot’s segments and linkers, this robot can generate unidirectional movement (crawling in one direction), which has been challenging to achieve in previous soft robots.
Funding and commercial potential
The project has received the Emerging Frontiers and Multidisciplinary Activities (EFMA) grant from the US National Science Foundation. Johns Hopkins has already created successful products in this field. Back in 2021, they developed Theragrippers, tiny, dust-sized devices that deliver medicine directly to the intestines.
Conventional therapies can only provide 2 to 3 hours of drug retention. In contrast, Theragrippers stick to the colon up to 24 hours, leading to much higher drug exposure with a single dose. Judging by this track record, this new type of multisegmented robot will be helpful for untethered, precise movement in tight spaces.
Finding these inventions and their patents isn’t always easy using Google search. You often get an overwhelming number of results with only surface-level information. That’s where an innovation scouting tool like Slate helps out.
Slate takes a simple query and starts scouring through hundreds of patents, research papers, and other publications to curate highly relevant results for you to explore. Here’s what it came up with when asked, “Give me the latest inventions in soft gelatinous robots used as drug delivery systems.“
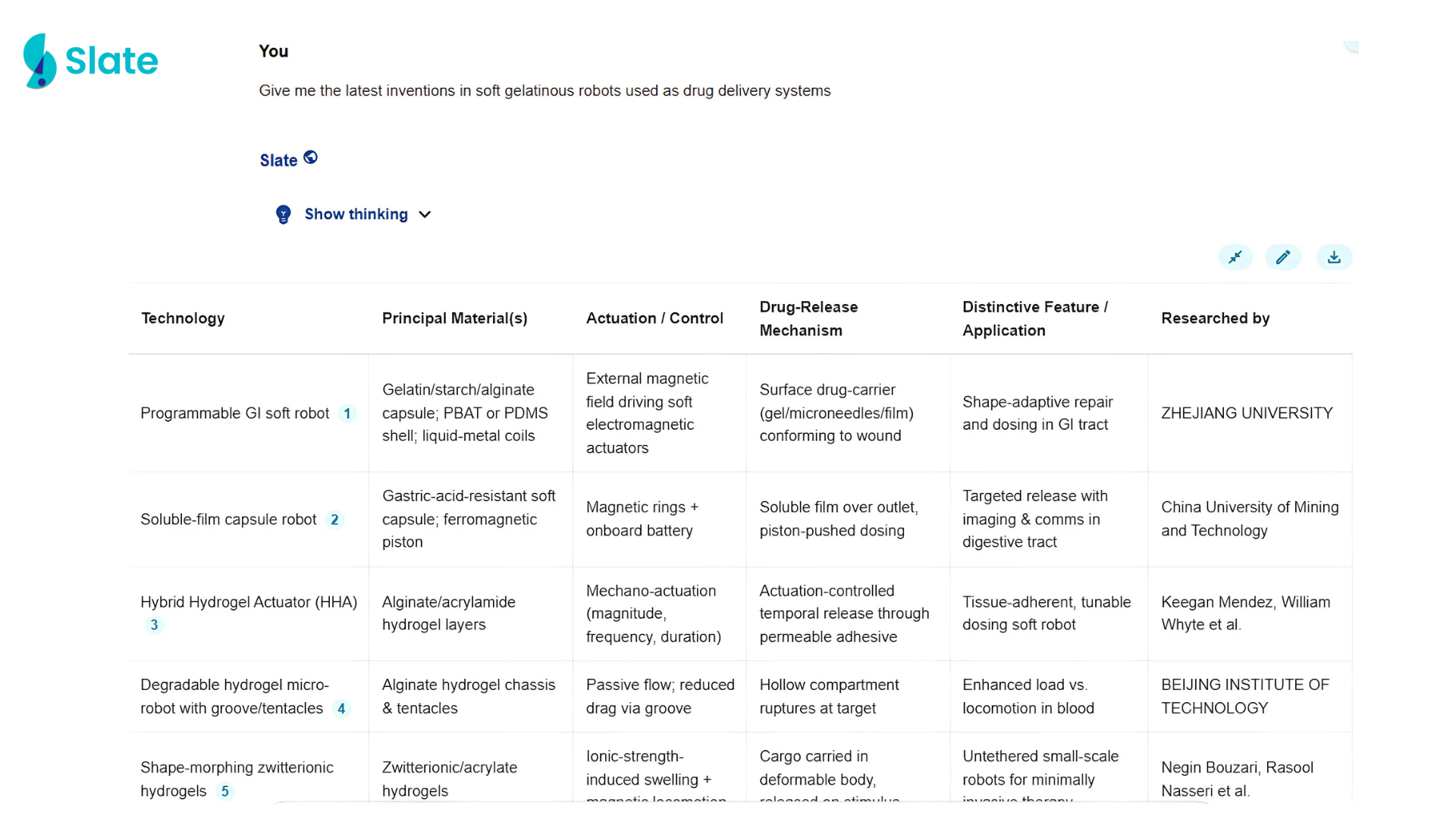
It also allows you to “chat” with these research papers to find specific details easily. Try out Slate yourself using the simple form below.
Eli Lilly’s nanoparticle solution treats nervous system disorders by passing through the blood-brain barrier.
Most treatments for Central Nervous System (CNS) disorders struggle to cross the blood-brain barrier and distribute the medications evenly throughout the brain. Eli Lilly & Co. and the University of Santiago have developed a solution using nanoparticles that can pass this barrier.
Their patented nanoformulation supports delivery in various ways, such as through the back of the neck, directly into the cerebrospinal fluid, or through the nose. This allows doctors to tailor treatments to each patient. Additionally, the particles have an average diameter ranging from 25 to 250 nm, enabling them to cross the blood-brain barrier (BBB). They also exhibit long-term stability even at low temperatures (e.g., stored at 4°C for a month).
This innovation enables the delivery of therapeutic agents to areas of the brain that were previously inaccessible. These nanoformulations can deliver a variety of therapeutic agents, such as RNA agents (e.g., mRNA, siRNA), targeting CNS-associated diseases. They can help in gene silencing or gene activation to treat conditions like Alzheimer’s, Parkinson’s, and epilepsy. Additionally, the ability to customize the formulation with different lipids, oils, and surfactants, as well as the inclusion of various therapeutic agents, adds to the practicality and adaptability of the innovation for a range of conditions.
On a similar note, there’s a nanobiotech startup called Nanotransfer that creates nanoparticle-based delivery systems for gene therapies. Their platform is designed to treat genetic diseases by delivering DNA, RNA, or gene-editing tools, such as CRISPR, into target cells. Their process enables them to select an appropriate nanoparticle as a vehicle, based on the size of the gene and the target tissue.
Manufacturing these nanoparticles is simple. Their particles avoid degradation by escaping cellular compartments, making them more sustainable and versatile. Unlike traditional viral vectors, their solution is non-toxic, non-immunogenic, and re-administerable, making it safer and more scalable.
Funding and commercial potential
Eli Lilly has already taken the next step to bring this into the market. The company entered a $1.4 billion partnership with Sangamo Therapeutics in 2025 for delivering genomic medicines to the CNS, using the same delivery technology.
Challenges to Foresee with Nanotechnology and Soft Robots
| Challenge Area | Gelbot | Nanoparticle CNS |
| Scale-up | Complex 3D printing | Lipid formulation control |
| Targeting | Passive, stimulus-driven | Active, customizable delivery |
| Regulatory Path | Device + drug combo | Drug + gene therapy hybrid |
| Safety Concerns | Biocompatibility, degradation | Neurotoxicity, immune response |
- The movement of these soft robots depends on factors such as temperature, pH, or light, which can vary unpredictably within the human body.
- Incorporating drugs or sensors inside the robot without disrupting its locomotion or biocompatibility is a tricky balance.
- Since these gel materials are sensitive to heat and chemicals, they’ll complicate sterilization and packaging processes.
- FDA may regulate these robots as a combination product (device + drug), requiring dual pathways.
- Crossing the BBB with nanoparticles is still not standardized. Each formulation could face a case-by-case review.
- Scaling production on these lipid-based nanoparticles will require stricter process controls to ensure consistency across batches.
- The nanoparticle solution must demonstrate no accumulation in brain tissue and minimal off-target effects to avoid regulatory scrutiny.
- Additionally, these RNA-based payloads may be subject to further scrutiny under gene therapy guidelines.
The path ahead
Soft robots and nanotechnology are among the most promising choices for innovators developing safe and effective drug delivery systems. For instance, Concept Medical has developed a system called Magic Touch. It’s a Sirolimus-Coated Balloon (SCB), designed to treat narrowed or blocked arteries. It’s already in commercial use in Europe and Asia.
Similarly, Korean medical device company Genoss and Ajou University have developed a microgel for sustained drug release, achieving 83% release over a 14-day period. Korea Medical Device Development Fund is supporting this research.
There are many more such emerging innovations waiting to be explored. Our report provides more detailed information on additional research areas, including lipid nanoparticle (LNP) formulations and cancer stem cell targeting, along with relevant patent data. Fill out the form below to gain full access to the report.