The European Commission has recently postponed the ban on TiO2 in medicinal products due to the lack of viable alternatives. This move highlights the overreliance on TiO2 and the need to transition away from it. According to EMA’s Article 57 database, over 91,000 medicinal products in the European Economic Area (EEA) contain TiO2. A global ban could affect hundreds of thousands of SKUs, requiring expensive reformulation, re-approval, and supply chain adjustments.
But manufacturers have faced significant challenges with whiteness, UV degradation, and safety profile in developing a new alternative for titanium dioxide (TiO2). Regulatory authorities, such as the EMA, emphasize that replacements fail to match TiO2’s performance in critical areas, including uniform opacity, light protection, and long-term stability. They estimate that reformulations could take between 4 and 6 years per product, potentially spanning 7 to 12 years for a complete portfolio shift.
While most R&D leaders have already trialed early candidates, such as calcium carbonate and cellulose-based coatings, they have found them to be incomplete. This report discusses some new, efficient alternatives to TiO2 in the pharmaceutical industry that do not have show-stopping limitations. In addition to this report, our experts conducted a webinar on TiO2 alternatives. Below is the full report of our research. Please fill out the simple form to access it.

Why Viable TiO₂ Alternatives Are So Rare?
Replacing titanium dioxide in pharmaceuticals is more than just a regulatory hurdle; it’s a complicated process that affects formulation, manufacturing, and compliance.
While many of the weaknesses in existing alternatives are known, what’s less obvious is how closely linked the challenges are. A safer ingredient may not work well in high-speed machines. One that looks white enough might not be compatible with standard coating systems. And even strong lab results often fail to scale due to cost, stability, or lack of regulatory support.
Because of this, many pharma companies have delayed action. They know that a change is needed, but are unsure whether the trade-offs are worth it. But that’s starting to shift. Promising new alternatives are emerging that break this pattern, offering real potential for TiO₂-free products without the usual compromises.
Sustainable and scalable Titanium Dioxide Alternatives for Pharmaceuticals
New Cyphochilus Beetle-Inspired Pigment Maintains High Whiteness.
Seprify AG (formerly Impossible Materials), a Swiss deep-tech spin-out from the University of Cambridge and University of Fribourg, has developed a 100% cellulose-based white pigment inspired by the ultra-white Cyphochilus beetle. It achieves high opacity through light-scattering microstructures rather than chemical additives.
The company utilizes its in-house, patented extraction & separation technology process, which physically tunes cellulose functionality without chemical modifications or harmful processing. It uses sustainable feedstocks, including wood pulp and agrofibers. The resulting pigments (SkyWhite™, SnoWhite™, CelluloseWhite™) have demonstrated opacity and whiteness comparable to TiO2, and are positioned for use across cosmetics, food, coatings, and potentially pharmaceutical formulations.
Seprify has scaled production from kg to ton-scale, securing key non-dilutive funding (CHF 150K and CHF 2M) to enable pilot manufacturing and commercial collaborations.
Seprify actively participated in Vitafoods Europe (May 2025) to engage with potential partners from the nutraceuticals and pharmaceutical community.
The startup claims that over 60 companies have reached out and shown strong interest in applying their materials. This indicates apparent market demand, strong validation of the technology’s relevance, and significant potential for partnerships or adoption. Seprify’s TiO2 alternative has a strong early commercial traction.
Ease of Integration with existing systems
Seprify’s cellulose-based pigment is engineered for seamless integration into existing manufacturing systems. It is compatible with standard mixing, coating, and extrusion equipment, requiring no significant changes to infrastructure. The pigment’s particle size and dispersion behavior are optimized to match TiO2’s performance, allowing formulators to maintain visual and functional quality while transitioning to a safer, sustainable alternative.
Seprify also supports partners with technical documentation and formulation guidance, making it a practical choice for companies seeking to phase out TiO₂ without sacrificing efficiency or compliance.
Seprify’s Cellulose-Based Pigment vs Titanium Dioxide
| Feature | Titanium Dioxide (TiO2) | Seprify Cellulose-Based Pigment |
| Safety Profile | Under scrutiny for potential carcinogenicity (EU banned in food) | Non-cytotoxic, plant-derived, larger particle size avoids nanoparticle-related concerns |
| Regulatory Status | Banned in EU food; still permitted in pharma (under review) | Designed to meet pharma and food safety standards |
| Source Material | Mineral-based, non-renewable | Cellulose from biomass (e.g., wood pulp, agri waste) |
| Environmental Impact | High carbon footprint, mining-intensive | Low-impact, renewable, supports the circular economy, and provides substantial CO2 reduction. |
| Optical Performance | High whiteness, but requires thick layers | It achieves whiteness with thin layers |
| Scalability & Cost | Widely available, but facing supply and cost pressures | Scalable from waste streams; competitive pricing potential |

Zinc and Calcium Capsules Outperform HPMC and Gelatin in Stability and Whiteness
Qualicaps, a Roquette subsidiary and one of the world’s leading capsule producers, has positioned itself as a pioneer of TiO2 replacement with its 2023 launch of Quali-V® TiO2-free (HPMC) and Quali-G™ TiO2-free (gelatin) capsules. Both rely on a patented combination of zinc oxide (ZnO) and calcium carbonate (CaCO₃) as opacifiers, delivering intense whiteness, opacity, and mechanical performance comparable to that of TiO₂ capsules, while maintaining high-speed machinability and 24-month stability.
Quali-V® capsules are fully compliant with major pharmacopeias (USP, Ph. Eur., and JP) and are supported by validated Drug Master Files (DMFs) in the US and Canada. Moreover, they meet stringent ISO 9001 and ISO 14001 certification standards, ensuring suitability for global pharmaceutical use.
Quali-V capsules are already used in commercial pharmaceutical and nutraceutical products, particularly those requiring moisture-stable, animal-free dosage forms. The product is widely available across multiple global markets, leveraging Qualicaps’ manufacturing legacy and reach.
Ease of Integration with existing systems
Quali-V® TiO2-free (HPMC) and Quali-G™ TiO2-free (gelatin) capsules by Qualicaps offer high compatibility with existing pharmaceutical manufacturing workflows. They have been validated to maintain excellent machinability on high-speed filling and sealing machines, matching TiO2-containing formats in terms of production efficiency. They also deliver comparable dissolution and disintegration profiles and maintain stability for 24 months.
Quali-G™ TiO₂-Free (Qualicaps) vs Titanium Dioxide
| Feature | Titanium Dioxide (TiO₂) | Quali-G™ TiO₂-Free (Qualicaps) |
| Safety Profile | Under scrutiny for potential genotoxicity and nanoparticle risks; banned in the EU food sector | Designed to meet EU and global pharma standards; supports proactive regulatory compliance. |
| Regulatory Status | Banned in EU food since 2022; still permitted in pharma pending EMA review (due April 2024) | Designed to meet EU and global pharma standards; supports proactive regulatory compliance. |
| Source Material | Mineral-based (rutile or anatase forms of TiO₂) | Plant-derived and synthetic excipients; avoids mineral extraction |
| Environmental Impact | High carbon footprint due to mining and processing; non-renewable | Lower impact; avoids mining, supports clean-label and sustainable sourcing |
| Optical Performance | Excellent whiteness and opacity; industry benchmark | Comparable whiteness and coverage using alternative pigments and film-forming agents |
| Scalability & Cost | Widely available and cost-effective; entrenched in supply chains | Slightly higher cost due to reformulation; scalable with existing capsule infrastructure |
How does it solve the problem with TiO2?
- The absence of nanomaterials in both formulations – Quali-V® TiO2-free and Quali-G™ TiO2-free capsules, containing the new opacifier.
- Quali-V capsules, formulated with the new opacifying agent, show the second-highest opacity after TiO2.
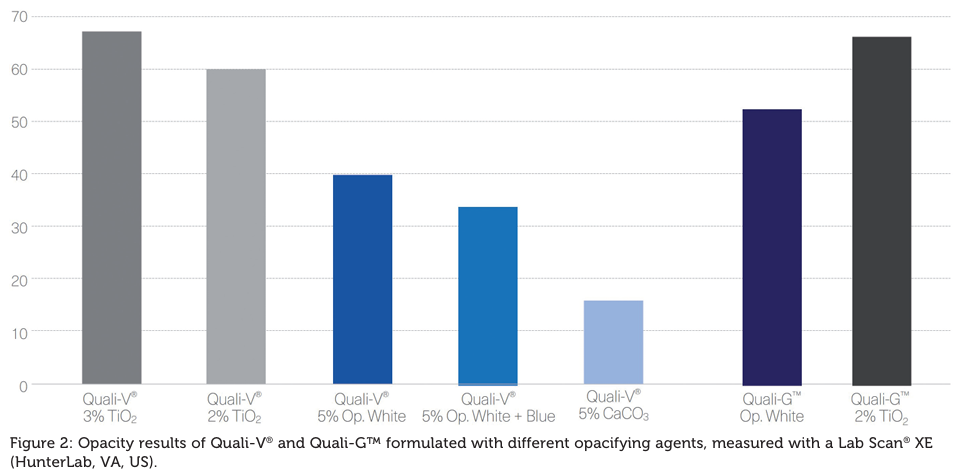
- Dissolution Testing for immediate release dosage forms”, in which the acceptance criteria are stated as no less than 80% of the API dissolves in less than 45 minutes. Quali-V TiO2-free and Quali-V with TiO2 dissolution profiles are comparable at both pH levels, 1.2 and 6.8.
- The disintegration test results for Quali-V TiO2-free capsules showed a disintegration time of no more than 15 minutes, meeting the acceptance criteria. Similar dissolution and disintegration test results have been obtained for Quali-G TiO2-free capsules compared to Quali-G capsules.
- The current shelf life for both Quali-V® TiO2-free and Quali-G™ TiO2-free products is 24 months.
- It has good machine compatibility, making it suitable for automatic, high-speed filling and sealing operations, while inheriting Qualicaps’ standard manufacturing processes with no change in fill equipment.
Xylitol combined with Magnesium Stearate (MgSt)
Roquette, a global leader in plant-based excipients, has introduced a novel TiO2-free tablet coating technology that replaces traditional titanium dioxide with xylitol (XYLISORB 300) and magnesium stearate (MgSt). This crystallization-based hard-coating method achieves near-TiO2 levels of whiteness (~1% TiO2 equivalent), enhanced gloss, and smooth tablet surfaces, all using existing coating equipment and without the use of heavy metals.
Roquette has commercially launched the following products:
- Tabshield is a ready-to-use film coating range featuring HPMC, PVA, and methacrylic acid polymer systems in TiO2-free formats (immediate- and enteric-release, customizable colors) for pharmaceutical and nutraceutical applications. It has been developed in partnership with Kofitech, a Korea-based company specializing in film-coating materials and the owner of the Tabshield brand.
- ReadiLYCOAT is also a ready-to-use film coating platform with multiple variants, including a plant-based film coating system and an xylitol-based sugar-free coating system, among others. The xylitol-based sugar-free sugar coating system, i.e,. The XC variant (mixture of xylitol, LYCOAT® hydroxypropyl pea starch, and magnesium stearate) offers sugar-free coatings with smooth finishes and significantly reduced processing times.
Roquette has partnered with Kofitech Co., Ltd., leveraging its Tabshield® brand to combine coating innovation with industry expertise and commercial rollout.
The startup presented its technology at the 5th European Conference on Pharmaceutics in March 2025 in Porto, demonstrating the feasibility of ReadiLYCOAT XC hard-coating colored tablet cores to achieve superior whiteness via MgSt crystallization.
Ease of Integration with existing systems
Roquette’s XYLISORB 300 xylitol, combined with magnesium stearate, offers a TiO₂-free coating solution that integrates well with standard pharmaceutical coating processes. While it may require slight adjustments to drying times or film thickness, it is compatible with conventional pan coaters and fluid bed systems.
The formulation utilizes familiar excipients, making it easier for teams to adapt without requiring significant workflow changes. Roquette provides technical support and documentation to help manufacturers update their SOPs and meet regulatory requirements, making this solution a flexible yet scalable alternative for companies seeking to phase out TiO₂ in coated tablets or capsules.
Xylitol Combined with Magnesium Stearate (MgSt) Achieves Near-TiO₂ Whiteness without Heavy Metals
| Feature | Titanium Dioxide (TiO₂) | XYLISORB® 300 + Magnesium Stearate (Roquette) |
| Safety Profile | Under scrutiny for potential genotoxicity and nanoparticle risks; banned in EU food | Non-toxic, non-nano; avoids genotoxicity concerns and supports clean-label compliance |
| Regulatory Status | Approved in pharma but banned in EU food; under EMA review for medicines | Designed to meet EU and global pharma standards; supports TiO₂-free reformulation |
| Source Material | Mineral-based (rutile or anatase forms) | Xylitol from plant-based polyols; MgSt from fatty acids (vegetable or animal origin) |
| Environmental Impact | Mining-intensive, non-renewable; high carbon footprint | Lower impact; renewable sources; compatible with eco-conscious manufacturing practices |
| Optical Performance | Industry benchmark for whiteness and opacity | Achieves comparable whiteness with optimized MgSt ratios; crystalline coating structure |
| Scalability & Cost | Widely available and cost-effective | Compatible with existing equipment; slightly higher cost but scalable for hard coatings |
Conclusion
The industry’s transition away from titanium dioxide is no longer a matter of scientific possibility but of strategic timing. While the European Commission has postponed a formal ban on pharmaceuticals, regulatory and consumer pressure make TiO2’s long-term future increasingly uncertain. Solutions from innovators like Seprify (cellulose-based pigments), Qualicaps (TiO2-free capsules), and Roquette (xylitol–magnesium stearate coatings) demonstrate that technically viable, scalable, and cleaner-label alternatives are already emerging.
Each comes with trade-offs in terms of cost, adoption speed, or IP protection, but collectively they point to a clear direction of progress. For R&D and open innovation leaders, the imperative is to move forward now by piloting alternatives, building supply partnerships, and validating performance in real-world formulations.
As a next step, our experts recommend that R&D leaders understand how the EU’s ban on TiO2 will impact their pharma product development. A recent webinar hosted by our experts reveals precisely that, and more. Please use the form below to access the full recording along with our exclusive research data on titanium dioxide alternatives.