The Mounjaro Patent Thicket: Patents expiring in 2036
More than 500 million people around the world are affected by diabetes, and this issue is intensified by a surging obesity crisis, particularly in wealthier nations.
In such a crisis, drugs like Mounjaro represent a highly lucrative opportunity for generic drugmakers to address the escalating medical demand on a global scale.
For the uninitiated, Mounajro® is the brand name of Tirzepatide, developed by Eli Lilly & Company. This medication is prescribed for managing Type 2 diabetes, addressing chronic weight concerns, and, notably, for addressing sleep apnea, although the latter is not an officially approved indication.
The drug was first approved in 2022 and is sold globally, including in the United States, Europe, Canada, Australia, Japan, and Korea.
Here is the timeline for which different countries approved Mounjaro for different diseases:
Mounjaro’s Approval Timeline
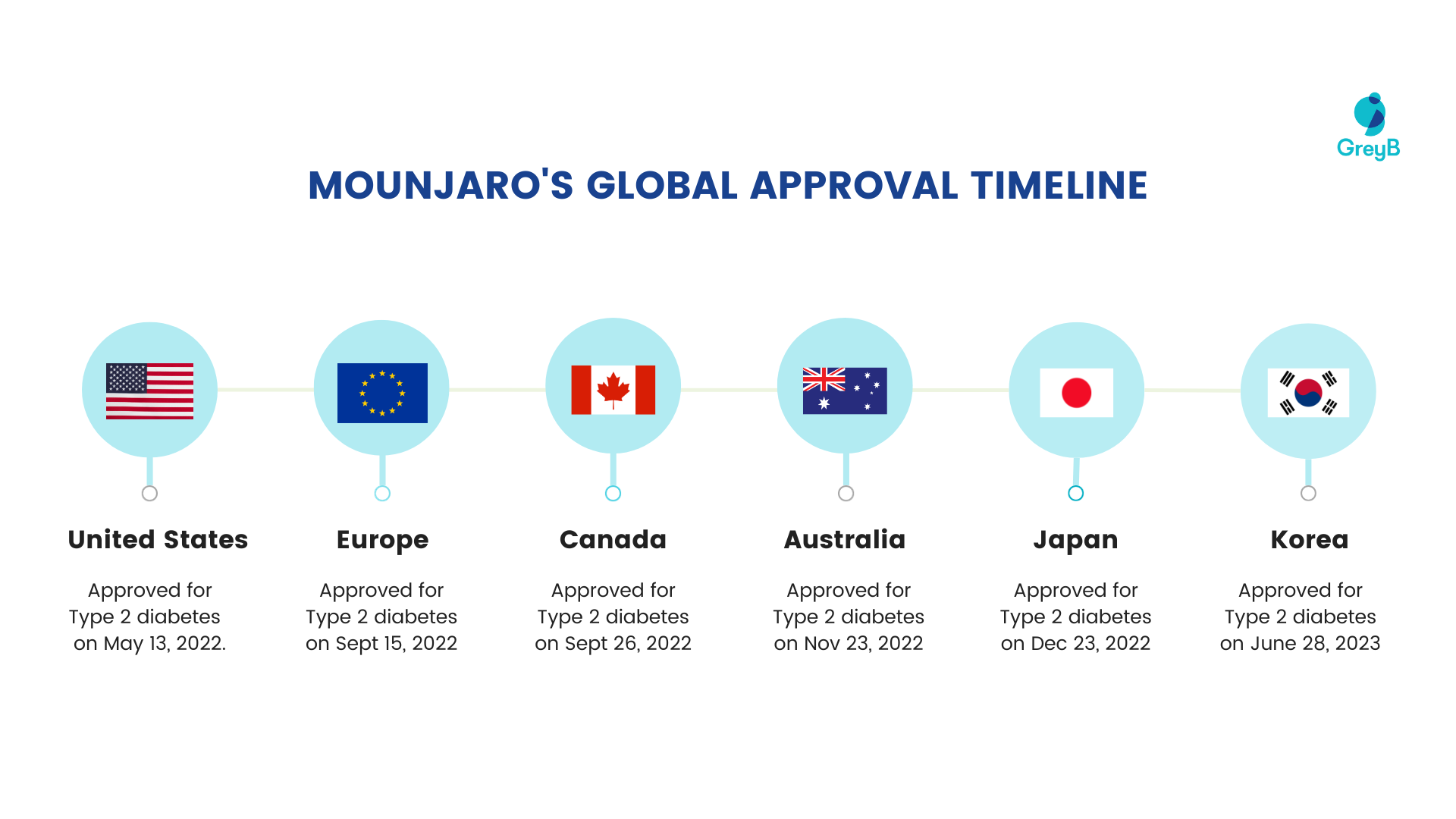
Tirzepatide received approval from the US FDA for chronic weight management on November 8, 2023. It is now available under the brand name Zepbound®, offering an approved solution for weight management.
Why is Mounjaro important to Eli Lilly?
In 2022, Mounjaro’s total revenue amounted to $203.2 million, experiencing a significant surge to $2957.5 million in 2023. Notably, third-quarter global revenue reached $1.41 billion, with a substantial $1.28 billion stemming exclusively from the US.
Meanwhile, the revenue outside the US was 132.4 million dollars.
This notable surge in sales can be credited to Tirzepatide’s innovative therapeutic application for chronic weight management. Marketed under the brand name Zepbound®, Tirzepatide’s sales are anticipated to reach approximately $2 billion in 2024, demonstrating its remarkable success despite having received approval only in November 2023.
Moreover, Tirzepatide is a blockbuster drug for type 2 diabetes, currently lacking a generic version in the market. The molecule patent for Tirzepatide will expire in 2036, allowing generic manufacturers to produce their versions. However, the launch of generic formulations is restricted until 2039 due to four active formulation patents.
To protect its position and investment, like every drug company, Eli Lilly has strategically filed multiple patents around the drug, forming a patent thicket.
Here’s how it looks:
| Indications | Sleep Apnea | Type 2 Diabetes/Glycemic Control | Chronic Weight Management |
| Composition of Matter | US9474780B2 Expires on Jan 05, 2036 US20220135639A1 Under Application Stage WO2023028466A1 Under Application Stage | ||
| Indication/ Method of Treatment | WO2022271611A1 Under Application Stage | – | – |
| Dosage Regimen | – | US20230355719A1, CA3208208A1, US20210338781A1, US2020023040A1(Once weekly) Under Application stage | – |
| Formulation | US11357820B2 Expires on June 14, 2039 PK202300432A0 Under Application stage | ||
| Manufacturing | US20220135639A1, WO2021158444A2 Under Application stage | ||
| Other (Delivery Device, etc.) | US9402957B2 Expires on June 29, 2031 | ||
As top blockbuster drugs approach patent expiration, strategic ANDA filings are critical to secure early mover advantage. Download the Pharsight Digester report now for a deep dive into the patent strategies of the top 5 blockbuster drugs nearing expiration.
GreyB’s Analysis
With promising trial results, there is likely to be an enormous demand for Mounjaro. If development continues successfully, this drug could profoundly impact global diabetes care in the coming decades.
Therefore, tracking its patent expirations beforehand can not only help evaluate business opportunities but also help refine your market entry strategies.
Elixir helps you stay vigilant and monitor activities related to the patents, giving you a headstart in the generic market.
Try Elixir today!
Authored By: Shadab Riyaz, Life Sciences
Edited By: Ridhima Mahajan, Marketing




