According to Deloitte, the biopharma industry generates 300 million tons of plastic waste annually, much of which is single-use.
“The ideal destination for plastic waste is not incinerators or landfills, but a return to supply chains,” Jacqueline Hollands, Global Manager of Recycling and Innovation for MilliporeSigma. “However, the plastics discarded in biomanufacturing are uniquely difficult to recycle.”
In 2025, three significant challenges stand out for the pharmaceutical packaging industry.

Key challenges in pharmaceutical packaging include balancing sustainability with drug protection, as eco-friendly materials must meet stringent safety standards; improving anti-counterfeiting and supply chain security, as counterfeit drugs persist despite the implementation of track-and-trace solutions; and enhancing patient usability and compliance, as packaging must cater to diverse patient needs while adhering to complex regulations.
This article thoroughly examines the pressing challenges, assessing their impact on the industry and the latest available solutions.
Interested in the Pharma Packaging Trends 2025 Report. Fill out the form to get the report:

Challenge 1: Sustainability with Safety Challenge
There is intense pressure to make pharma packaging more sustainable without compromising drug safety or regulatory compliance.
Medicines are sensitive products that require high-barrier, inert packaging materials, such as plastic, glass, or aluminum foil. The primary challenge is to maintain eco-friendly packaging while meeting stringent requirements for protection, stability, and child safety.
Regulatory bodies such as the European Medicines Agency mandate that any change in packaging must not affect the product’s quality. Therefore, companies cannot simply switch to a greener material if it risks interaction with the drug or a shorter shelf life.
As the Association of the British Pharmaceutical Industry notes, any changes aimed at improving sustainability must ensure that there is no increased risk to patient safety or product contamination.
This challenge is significant because pharmaceutical packaging contributes substantially to medical waste streams.
Consumers and policymakers in North America and Europe call for more environmentally friendly solutions. For example, blister packs that combine plastic and foil are challenging to recycle, resulting in millions of packages being sent to landfills annually.
Emerging Solutions
Companies are exploring alternative materials, such as plant-based plastics, recyclable mono-material packaging, and reusable packaging systems. Many pharmaceutical companies are transitioning to bioplastics or paper-based alternatives, replacing petroleum-based plastics.
One promising solution is mono-material packaging, which uses a single recyclable polymer instead of layered materials. A partnership between packaging firms Perlen and Etimex aims to introduce a polypropylene mono-blister pack, addressing the demand for an eco-friendly blister that can protect tablets.
“We are responding to our customers’ requests and expanding our existing product range. The cooperation with Etimex enables us to offer a tried and tested, fully recyclable solution based on polypropylene from a single source.” Lars Kirchhoff, CSO, Perlen Packaging.
Another innovation is the use of renewable materials in traditional formats. For instance, UPM Biochemicals, Selenis, and Bormioli Pharma jointly developed the industry’s first medicine bottle made from wood-based bio-PET, a renewable plastic. This bottle has the same form and function as a conventional PET bottle, but has a lower carbon footprint.

Michael Duetsch, Vice President of Biochemicals, UPM, explains: “Our pioneering collaboration demonstrates that renewable, wood-based biochemicals can deliver the same high quality as fossil-based virgin materials, with significant environmental benefits.”
Such developments show that viable solutions are emerging, at least in prototype or early commercial stages.
Several big pharmaceutical companies have also formed coalitions to tackle this issue. In Japan, four major pharmaceutical companies— Astellas, Eisai, Daiichi Sankyo, and Takeda —collaborated to share knowledge and promote more environmentally friendly packaging.
What are the challenges with the existing solutions?
While some sustainable packaging solutions are available, many are not yet widely implemented. Recyclable or bio-based packages are on the market, but each must undergo rigorous testing to be approved for pharmaceutical use.
For example, bio-based PET bottles are already commercially available, whereas fully compostable pill blister packs are still in the pilot stages.
Companies must validate that new materials will not interact with medications or degrade their protective properties. Moreover, switching to new packaging can be costly and require regulatory filings.
The key barrier is ensuring that the latest sustainable materials match the performance of traditional packaging, for instance, maintaining moisture/oxygen barriers and stability over a drug’s shelf life.
One can easily find innovation in moisture barrier materials or oxygen barrier layers through Slate.
A patent by Proampac discusses a cellulose-based multilayer packaging. An oxygen barrier layer of Polyvinyl alcohol (PVOH) is attached as the innermost, heat-sealable layer. A moisture barrier layer is added, either between the substrate and the oxygen barrier or on the outer surface of the substrate.
Want to know more about such innovations? Explore Slate.
Alcohol Packaging Challenges and Solutions
Challenge 2: Anti-Counterfeiting and Supply Chain Security
Counterfeit and substandard medicines remain a serious global issue, and packaging is at the forefront of defense.
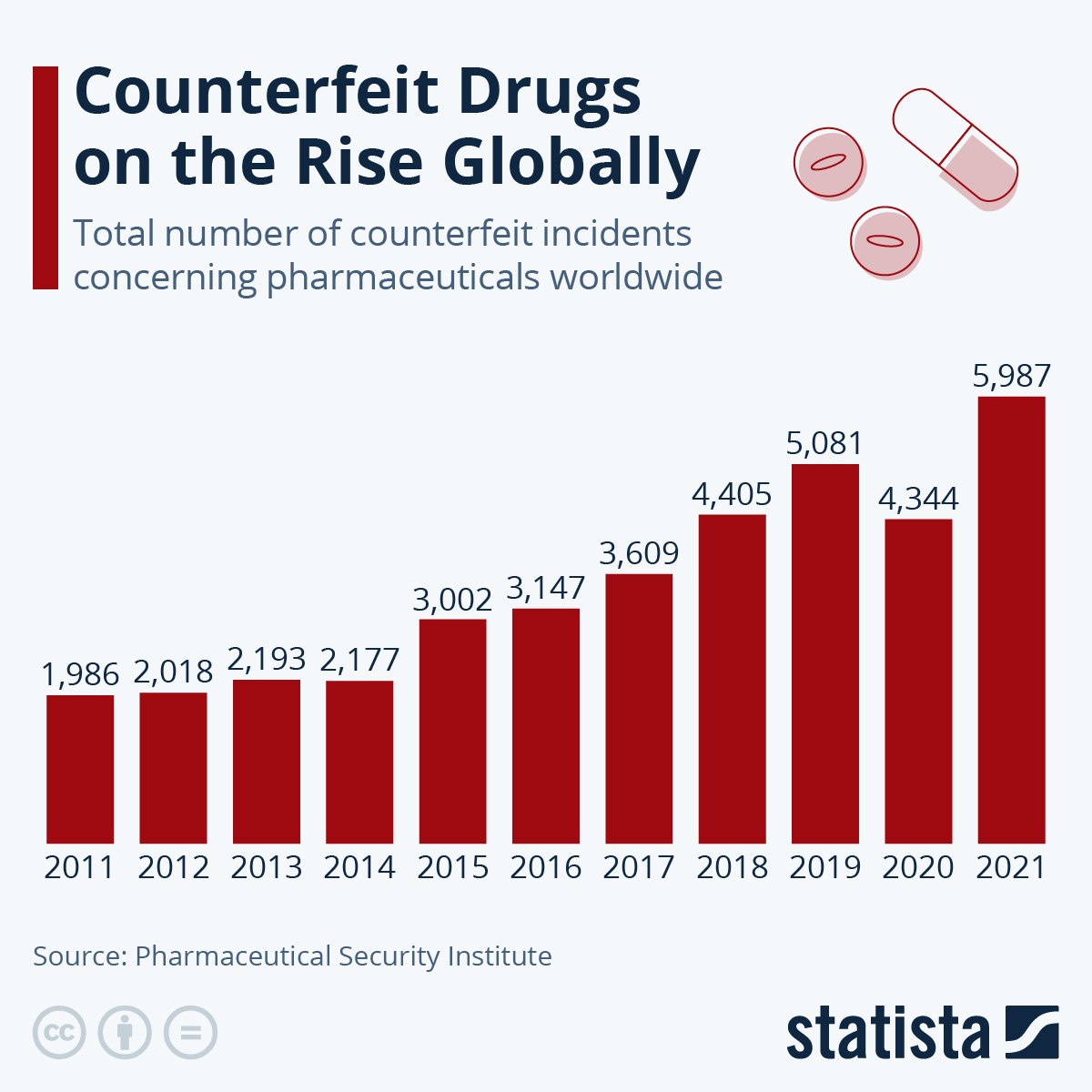
Globally, counterfeit drug sales amount to an estimated $83 billion annually, indicating the scale of illicit trade that adequate packaging security needs to thwart.
Regulative measures have been implemented in North America and Europe. The EU Falsified Medicines Directive and the US Drug Supply Chain Security Act (DSCSA) require unique identifiers (serial numbers) on each saleable package to enable tracking and verification.
Implementing these systems has been significant for R&D and operations teams over the past decade.
Even with laws in place, counterfeiters can adapt quickly.
Online pharmacies and global e-commerce add another layer of risk. A report by AstraZeneca states that over 50% of medicines sold online from illicit sites are counterfeit.
Existing Solutions
Serialization has been the primary solution across the industry. This involves assigning each product pack a unique code, commonly referred to as a barcode, that can be scanned and logged at every point in the supply chain. This creates an electronic pedigree for each pack, making it far more challenging for fake products to go undetected.
Beyond serialization, pharmaceutical companies are developing advanced anti-counterfeiting features, including tamper-evident seals and blister packaging that indicate if a package has been opened or altered.
Furthermore, there are:
- Holograms and color-shifting inks on labels that are hard to replicate
- embedded RFID or NFC tags that pharmacies can scan to confirm a product’s origin
- and even forensic methods, such as microscopic taggants or chemical markers in packaging materials.
One cutting-edge approach is using blockchain technology to create an immutable record of product movement. The FDA even conducted pilot programs with manufacturers (Merck), distributors, and retailers (Walmart) to verify drug supply chains using blockchain technology.
Another innovative solution is offered by the tech startup TruTag, which has developed tiny, edible microtags that can be integrated into pills or packaging coatings. These tags contain unique codes that are readable by optical scanners, providing covert authentication.
Established packaging security companies, such as Systech (now part of Markem-Imaje), have introduced solutions that turn a printed barcode into a digital “fingerprint.” By scanning the code, not only is the number read, but also the microscopic print pattern, verifying that it is an original print and not a counterfeit copy. These solutions demonstrate that multiple layers of defense can be technically incorporated into the packaging.
What are the challenges with the existing solutions?
The challenges preventing mainstream adoption of the most advanced tools are often cost and complexity. Because adding RFID/NFC chips or sophisticated taggants to every package can be expensive, companies reserve these for high-value drugs.
Additionally, supply chain partners require infrastructure to read and share data—a fully blockchain-based system or a ubiquitous RFID network necessitates industry-wide coordination, which is still in progress.
When introducing new technology, regulatory and privacy considerations are also present.
Another challenge is global harmonization. While North America and Europe have robust systems, many other markets are just beginning to require serialization, and not all systems are interoperable.
Counterfeiters exploit the weakest link. Therefore, until global security is achieved, the threat persists.
The current trajectory suggests that serialization and tamper-evident features will remain standard, and additional layers will gradually become more feasible as costs decrease.
Partnerships are key here. Pharma companies work with technology providers in consortia.
For instance, the MediLedger consortium in the US has brought together top pharmaceutical companies and distributors to pilot blockchain-based track-and-trace solutions.
Challenge 3: Enhancing Patient Usability and Compliance
The third major challenge is making packaging more patient-centric — easy to use, accessible, and supportive of adherence. Pharmaceutical packaging has traditionally been designed with the primary goal of safety and compliance, sometimes at the expense of user-friendliness.
Poor packaging design can lead to patients skipping doses or making errors during administration. Thus, companies must improve the packaging design to improve the patient experience while complying with all safety, child-proofing, and informational regulations.
The significance of this challenge is high. Non-adherence to medication is estimated to cost healthcare systems billions and is associated with thousands of preventable hospitalizations annually.
Existing Solutions
There are solutions to the above challenges, such as calendar blister packs or clearly labeled dose packets, which can remind and guide patients.
In 2025, there is a greater recognition that packaging is an integral part of the product’s user interface.
Pharmaceutical packaging designers are exploring various approaches to enhance the physical design. It’s creating blister packs with peel-off foil for individuals with limited dexterity, incorporating tactile cues for the visually impaired, or designing child-resistant and senior-friendly caps.
Another approach is to personalize packaging according to the patient’s regimen. A notable example is Amazon’s PillPack service, which delivers medications in personalized, perforated pouches labeled by date and time of dose. Instead of multiple pill bottles, a patient receives a roll of packets in chronological order.

This packaging innovation has improved adherence for patients with complex schedules. Community pharmacies and startups are adopting similar adherence packaging solutions to help patients take the correct medication at the right times.
Additionally, larger font sizes and clear layouts on labels and inserts are simple improvements implemented to make information more readable for elderly patients.
Digital technology also offers solutions by including QR codes on packaging. It links to instructional videos or schedule reminders, complementing the physical packaging.
Regulatory bodies in the EU are discussing electronic product information (ePI), which involves providing leaflet information digitally (via a QR code or app). This could allow for more user-friendly, interactive information while reducing the clutter of paper leaflets. Although this is still in the pilot phase, it is a trend to watch.
What are the challenges with the existing solutions?
These are viable solutions for patient-centric packaging. Many are in practice, though not yet universal.
Calendarized blister packs and compliance packaging are already used for specific medications, particularly in markets such as the US, for oral contraceptives or pharmacy-provided adherence packs. The benefit of such solutions is simply that they can significantly aid in tracking doses.
Personalization at scale is more challenging since it may require new packaging workflows or on-demand printing. Still, companies are experimenting with it in limited scenarios, such as clinical trial kits or specialty medications.
Regulatory compliance adds complexity – for example, child-resistant packaging is legally required for many drugs, so any easy-open feature must be carefully engineered to ensure safety is not compromised.
Many innovative designs undergo rigorous testing to ensure they are both senior-friendly and child-resistant, setting a high standard to meet. Moreover, implementing e-labeling requires regulatory approval and ensuring all patients can access digital info.

Innovative Packaging Startup Report
Download ReportNext Steps
Companies today often employ human factors engineering, which involves testing prototypes with patient groups to gather feedback, as part of the packaging development process. The challenge is integrating these user-friendly features without adding excessive cost or complexity while navigating the patchwork of regulations.
In 2025, a positive trend is emerging as packaging is slowly becoming more intuitive and supportive.
For example, some prescription blister packs now include concealed electronic trackers that log when a dose is removed. These trackers help monitor adherence in clinical trials and could eventually be used by patients or caregivers in the real world.
The current challenge to mainstream innovative compliance packaging is ensuring data privacy, cost-effectiveness, and the willingness of healthcare systems to adopt these tools. Nonetheless, the trajectory is that patient-centric design is moving from a secondary consideration to a core design principle for pharma packaging.
In addition to the above, companies also face other challenges, such as packaging for new therapies that require cold chain (e.g., mRNA vaccines that necessitate ultra-cold thermal shippers) and improving packaging line efficiency for cost control.
The COVID-19 vaccine rollout exemplified a packaging challenge that was overcome through innovation. Pfizer, for instance, developed suitcase-sized thermal shippers with GPS-enabled temperature sensors to distribute vaccines at -70°C.
Such advances show R&D’s ability to respond to urgent packaging challenges. While not detailed above, these aspects are discussed in the trends section for novel therapy packaging.
This is just a glimpse into the vast landscape of pharmaceutical packaging.
If you are seeking a solution for a particular challenge or want to explore the whole domain to get solution ideas, do reach out to us by filling out the form below:
How Can We Help You?
We support industry-leading R&D and Innovation professionals through complex problems. Describe your challenge, and let us bring clarity and expertise.